| Issue |
Matériaux & Techniques
Volume 112, Number 5, 2024
Special Issue on ‘Circular Economy initiatives and solutions in the steel sector’, edited by Valentina Colla and Ismael Matino
|
|
|---|---|---|
| Article Number | 503 | |
| Number of page(s) | 23 | |
| Section | Circular economy, recycling, reuse, sobriety | |
| DOI | https://doi.org/10.1051/mattech/2024026 | |
| Published online | 21 November 2024 | |
Review
Roadmap for recycling practices and resource utilization in the iron and steelmaking industry: a case studies
1
K1-MET GmbH, 4020 Linz, Austria
2
Acciaierie d’Italia S.p.A., 20151 Milano, Italy
3
ArcelorMittal Belgium NV, 1000 Bruxelles, Belgium
4
Butter Bridge BV, 1000 Amsterdam, The Netherlands
5
University of Oulu, 90570 Oulu, Finland
6
Politecnico di Milano, 20133 Milano, Italy
7
RINA Consulting − Centro Sviluppo Materiali S.p.A., 00128 Rome, Italy
8
Scuola Superiore Sant’Anna, 56127 Pisa, Italy
9
Institute of Intelligent Industrial Technologies and Systems for Advanced Manufacturing − National Research Council of Italy (STIIMA-CNR), 20133 Milano, Italy
10
Swerim AB, 97125 Luleå, Sweden
11
Tenova S.p.A., 21053 Castellanza, Italy
12
TU Dortmund University, 44339 Dortmund, Germany
13
Ghent University, 9052 Zwijnaarde, Gent, Belgium
* e-mail: lina.kieush@k1-met.com
Received:
6
February
2024
Accepted:
20
October
2024
This paper aims at providing an overview of the ways for residue valorization in the iron and steelmaking industry. The important role of recycling in iron and steelmaking as a cornerstone for achieving a cleaner and resource-efficient potential is described. Several research results concerning metals and metal oxides (scrap, scale), slags, dusts, process gases, and water recycling from the iron and steelmaking process are reviewed here, aiming to detect those research gaps that still need implementation and suggest potential approaches toward potential solutions. Through a comprehensive evaluation, several possibilities are provided to incorporate effectively in metallurgical processes the bio-based or bio-derived carbon materials, namely biomass, biochar, biocoke, and polymers from waste plastics to reduce the dependence on fuel and reducing agents from fossil sources, and therefore mitigating the related environmental impact of the steel industry. Eventually, this review highlights the importance of embracing circular economy (CE) principles in iron and steelmaking, along with considering opportunities for industrial symbiosis (IS) and exploring the role of digitalization and digital solutions in recycling practices.
Key words: Recycling / scrap / slag / biomass / biochar / plastics / iron and steelmaking
Publisher note: The article has been added in open access. The article has been corrected on 26 November.
© L. Kieush et al., 2024
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
1 Introduction
Steel production mainly involves two primary pathways: production from iron ore using the blast furnace-basic oxygen furnace (BF/BOF) route, which includes cokemaking and iron ore agglomeration processes, and secondary production from recycled steel in electric arc furnace (EAF) or induction furnaces [1,2]. The traditional BF/BOF route contributes to a majority of worldwide (70%) [3] steel production; it relies heavily on fossil fuels and reducing agents, resulting in significantly higher CO2 emissions (2.0–2.2 tCO2/tHM) [4–6] and energy consumption (13.0–14.0 GJ/tHM) than the EAF route (CO2 emissions of 0.3–1.3 tCO2/tHM) and (energy consumption of 4.0–10.0 GJ/tHM) [3].
Focusing on integrating the circular economy (CE) principles enables material production optimization, material consumption reduction, and contributions to emissions reduction in iron and steelmaking. The CE framework emphasizes minimizing waste and maximizing resource utilization by creating closed-loop systems where materials are reused, recycled, or remanufactured [7]. Applying CE principles in iron and steelmaking can enable efficient resource use and recycling [8]. For instance, steel scrap is highly recyclable and can be almost entirely reintegrated into the production of new steel [9]. This process can significantly reduce the demand for virgin raw materials, decrease energy consumption [10], and lower CO2 emissions compared to primary steel production from iron ore.
Moreover, the iron and steelmaking generates various by-products, such as slags, sludge, mill scale, flue dust, and tailings [11]. These by-products contain valuable materials like metals and minerals, and technologies for reusing these residues can reduce reliance on primary resources, decrease landfill volumes, and result in economic savings for steel plant operators. For instance, slag, a byproduct of the steelmaking process, can be reused in road construction, cement production, and as a raw material for producing fertilizers [12]. According to [13] 20.7 million tons of BF slag and 16.3 million tons of steelworks slag were produced and recovered in Europe in 2018. Among these steelwork slags, 52.3% is BOF slag, 34.9% is EAF slag, and 12.6% is other types of slag. Of this, 72.4% were recovered, 15.3% were stored temporarily, and 12.3% were sent to dumps. About 70.6% of recovered steel slags were used as an aggregate in concrete production. Road construction used 1.3%, while 4.5% went into hydraulic engineering. Fertilizer got 13.1%, and 10.5% was recycled in metallurgical processes or used elsewhere. Utilizing slag in these ways can reduce waste and offset the need for natural resources in other industries.
Optimizing the input of raw materials, such as iron ore, coal, and fluxes, can minimize waste and improve overall process efficiency. Furthermore, alternative carbon materials are of interest, especially given the reliance on fossil carbon materials (coal, coke, natural gas, etc.) in the iron and steelmaking industry. Due to environmental concerns, there is growing relevance in using alternative carbon materials, such as bio-based or bio-derived [14] or polymer-based [15]. However, due to the existing strict requirements for fuel and reducing agents, only partial replacement of fossil fuels is possible for several metallurgical processes, such as cokemaking or BF.
Water recycling and energy recovery should also be considered. Steelmaking is a water-intensive process, and implementing closed-loop water systems can allow for water reuse within the plant, reduce the need for freshwater, and minimize wastewater discharge. According to [16], approximately 90% of water used in the steel industry can be cleaned, cooled, and returned to the source. Additionally, it is possible to consider recovering and reusing energy generated during production processes, such as waste heat recovery systems in BF or by-product gases for electricity generation, contributing to overall energy efficiency and reducing reliance on external energy sources.
CO2 capture and sequestration (CCS) is a critical strategy for achieving net-zero emissions in steelmaking and other CO2-intensive industries [17]. Capturing CO2 emissions from steelmaking processes and utilizing them in other industrial applications, such as chemical production or enhanced oil recovery, can contribute to a CE by reducing net emissions. However, the high incremental cost and significant energy consumption associated with CCS deployment remain challenges to its practical scalability [18].
Establishing industrial symbiosis (IS), where waste or by-products from one industry serve as raw materials for another, can enhance resource or energy efficiency and reduce the environmental impacts [19]. Johansson et al. [20] demonstrated that IS offers opportunities for enhancing energy efficiency and reducing CO2 emissions in iron and steelmaking plants. For instance, this can be achieved by utilizing waste from other sectors as reducing agents or fuel and by integrating the process with district heating systems.
This review mainly focuses on the role of recycling in iron and steelmaking processes, emphasizing its significance in managing by-products or utilizing alternative carbon materials. Additionally, it examines approaches and strategies undertaken by the EU iron and steel industry concerning CE, IS, and metallurgical process efficiency.
The review is organized as follows: Section 2 examines the potential for recycling by-products generated from cokemaking and the main by-products in iron and steelmaking, such as slag, dust, and process gases, as well as the possibilities for recycling steel scrap. Moreover, the utilization of bio-based, bio-derived, or polymer-based carbon materials in iron and steelmaking is also considered. Section 3 discusses the circularity of non-steel resources and opportunities for IS. Section 4 explores the role of digitalization and digital solutions in recycling practices. Section 5 presents conclusions and outlook.
2 Recycling practices in iron and steelmaking
2.1 Recycling of the main by-products in iron and steelmaking
Firstly, during cokemaking, several valuable by-products are created, including solid and liquid wastes [21]. After coking, the coke is pushed out of the oven, and the dust produced is collected. The hot coke is then quenched, after which it is broken and screened into blast-furnace coke and breeze (coke lumps smaller than 20 mm). Coal and coke dusts can be utilized in the coal blend. Additionally, coke breeze can be used for iron ore sintering or pelletizing. The water and dust mixture from the quenching process is transported to a decantation basin. Once the coke oven gas is collected, water and tar are separated. In summary, the key by-products can include tar, pitch, ammonia liquor, benzene, elemental sulfur, sulfuric acid, ammonium sulfate, and polycyclic aromatic hydrocarbons (PAH)-filtered water. They can serve either as a product or can be utilized in a coal blend. Table 1 summarizes the options for recycling and utilizing the main by-products in iron and steelmaking.
Secondly, blast furnace slag (BFS) and steel slag (SS), generally called iron and steel slags, and are the main by-products of iron and steelmaking [23–25]. Figure 1 presents a schematic overview of iron and steelmaking processes [26], along with the types of slag produced at each stage. The primary types of slag generated in these processes are classified as follows: BF slag, BOF slag, EAF slag, and ladle slag. Additionally, in the wake of steel production, consequential by-products include various types of dust [27] and gases. Sintering plants have traditionally been used to recycle by-products in integrated steel plants. However, there are constraints on the materials that can be recycled if operational problems are to be avoided in the sinter plant or BF operations [24].
BF slag is a by-product of ironmaking, typically ranging from 220 to 370 kg of slag per ton of iron produced [28]. Additionally, BF slag is widely used as a supplementary cementitious material (SCM) [29]. Furthermore, it can be used in road construction, civil engineering works, fertilizer production, landfill daily cover, soil reclamation, etc. The composition of BF slag varies based on the ores, fluxing, and coke fed into the BF. Typically, silica, calcium, aluminum, magnesium, and oxygen comprise more than 95% of BF slag’s composition. Apart from other slags, BF slags have a relatively low iron oxide content [30] due to BFs’ highly reducing conditions.
During the production of molten steel, slag is formed due to chemical interactions between liquid oxides of silicon, manganese, phosphorus, iron, and lime or dolomitic lime, etc. Steel slags also contain iron oxide due to the oxidizing conditions in the BOF required for converting hot metal into molten steel and eliminating impurities by blowing oxygen. EAF slags similarly contain iron oxide. The main chemical composition of BF slag and steel slag is presented in Table 2.
It is estimated that approximately 10–15 wt.% of slag is produced per ton of molten steel [33]. Steel slag is usually subjected to metal recovery before its application outside the iron and steelmaking process. By applying mineral processing technologies such as crushing, grinding, classification, and magnetic separation, it is possible to produce steel scrap (90% of Fe) and iron oxide concentrate (Fe > 55%) from steel slag [30]. It contains about 30–50% CaO and 3–10% MgO and can be directly used as a flux in sintering, the BF, or the steelmaking process to substitute a part of limestone and dolomite. Furthermore, steel slags containing MnO can partially replace manganese ore, reducing costs and raw material demand. However, since some steel slags contain a notable amount of phosphorus and sulfur, which are detrimental to steel properties, and approximately 10–18% SiO2, the potential for direct use of steel slag in the iron and steelmaking process can be considered limited. The other part of steel slag (50–90% of the total steel slag) is subjected to metal recovery [34,35].
The methods for slag processing are different, depending on the cooling method, chemical and mineralogical composition of the slag, and its application. Various granulation methods, such as wet and dry technologies, can be employed to repurpose slag into a secondary raw material. For instance, wet granulation of BF slag ensures rapid cooling and high production rates, although it necessitates water treatment.
On the other hand, dry slag granulation presents advantages such as minimal wastewater and cost reductions and potential heat recovery, but it may encounter flow rate limitations. Diverse dry granulation solutions have specific EAF or LF slag granulation requirements. Effective slag cooling and granulation are fundamental for creating a mineral product suitable as a secondary resource for the construction sector (i.e., road, cement, mortar). Tenova developed technology for the dry granulation of the LF slag by forced air stream [36] under installation at the Pittini Group. This process does not use water as a slag’s coolant and permits sufficient fast cooling to obtain amorphous morphology for more than 90 wt.% of the solidified granules. The treated slag can be introduced in the economic circle as a fluxant for EAF or as raw materials for construction, thanks to the specific glass phase necessary to initiate hydraulic reactions.
To summarize, while BF slag is mainly devoted to replacing cement in concrete, steel slags are mostly used as a filler material in embankment construction or as aggregates in concrete due to the problems with volumetric expansion. Advancements in the slag quenching process have led to notable enhancements in the properties of steel slag for use as aggregates. Slags from BOF, EAF, and LF − commonly utilized in road construction, asphalt concrete, agricultural fertilizer, and soil improvement − can now be considered valuable resources for the production of cement clinker and mortar production [37]. The chemical properties of steel slag make it suitable for adsorbing H2S and metalloids from marine environments [32].
Additionally, a flowsheet-based model was developed by Petrucciani et al. [38] targeting the improvements in the slag valorization, which can be used for off-line investigation. This model is the basis for developing black-box hybrid based (physical+AI) models to be integrated into decision support systems (DSS), supporting optimal management and improved slag recycling and reuse. This work has been carried out under the EU-funded project entitled “Optimising slag reuse and recycling in electric steelmaking at optimum metallurgical performance through on-line characterization devices and intelligent decision support system − iSlag.”
In turn, Falsafi et al. [39] investigated the valorization opportunities for slag as a by-product of EAF steel production. A multi-criteria analytic hierarchy process (AHP) model has been developed to evaluate value chain configurations according to criteria such as technology, legislation, economic and environmental sustainability, and supply chain. The multi-criteria combined with the multi-expert modeling approach is balanced with the importance of different criteria from the actors’ points of view, such as steel producers and technology providers.
Matino et al. [40] showed how to optimize by-product reuse in Italian steelworks, focusing on internal recycling in the form of pellets, especially of by-products, whose main fraction is currently recovered in an internal quarry, namely BOF slag. The optimization model was created, described, and used to find the best solutions that allow cost minimization and increase environmental sustainability by considering the quality of the potential products, specifically pellets or fertilizer. Furthermore, it was found that a simulated by-product mixture was further improved by adding a suitable amount of binders, obtaining the following: BOF slag 65 wt.%, BOF sludge 27 wt.%, dolomite 1 wt.%, cement 7 wt.%. Moreover, a pelletization procedure was developed to achieve the highest yield and the best pellet features (namely size and compression strength) in an industrially viable way.
Nylund [41] found that EAF slag holds promise as a useful material for making cement. However, there are hurdles to overcome, like dealing with high levels of iron oxides and magnesia, along with the expenses linked to processing and transportation. Despite these challenges, EAF slag can still be put to good use as a supplementary cementous material or raw material for making cement clinker.
Concerning steelmaking, dust is obtained either in the form of dust from dry dust separation or sludge from wet dust separation units. Steelmaking dust contains heavy metals, including Zn, Pb, Cd, and Cr [42,43]. Hydrometallurgy and pyrometallurgy processes [44] can be applied to recover for metals from steelmaking dust. The majority of research has concentrated on using pyrometallurgy methods to recycle valuable metals [45]. In addition, EAF dust can be used as a construction material or as a filler in acoustic and thermal insulators [46].
The current “REcovering Metals and Mineral FRAction from steelmaking residues” (ReMFra) project, [47], aims to develop and validate a highly efficient pyrometallurgic melting and reduction TRL8 demonstration plant for recovering metals and minerals contained in a wide range of steelmaking residues, such as filter dust, scale, sludge, and slags, to obtain pig iron, iron-rich oxides, highly concentrated zinc oxide, and an inert slag. ReMFra consists of two main parts to be developed, improved, and tested at an industrial scale: the plasma reactor by Tenova and RecoDust (Fig. 2, [48]).
While the former will recover the coarse residues (scale, sludge, slag), the latter will target fine-grained dusts. The project allows for improving iron yield using recovered pig iron instead of new pig iron and replacing the iron ore with iron rich oxide. The recovery of concentrated ZnO and inert slag as by-products can provide a significant source of income and will contribute to overall carbon neutrality. Moreover, the process foresees using secondary carbon sources (i.e., waste plastics) as reducing agents to reach the full circularity. To conclude, ReMFra is expected to enable technological advances in the demonstrators involved and contribute to developing new standards, training programs, adaptation, and certification of industrial processes, thus facilitating the replication of the project.
Regarding the EAF off-gases, the Tenova iRecovery system [49] has more than thirteen running references worldwide. It comprises a heat recovery for steam generation (HRSG) with a radiant, evaporative cooled system (ECS) and convective section, the waste heat boiler (WHB). It can completely process the waste gas from 1700 to 200 °C, recovering up to 75% of the energy available in the waste gas, corresponding to approx. 20–25% of the total energy input in the EAF process. Pressurized water at boiling point feeds the ECS + WHB, which is converted into saturated steam through the heat exchanged with the waste gas. Saturated steam is the energy carrier for power generation or direct use in different thermal applications.
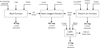 |
Fig. 1 Schematic of slag generation in a BF, BOF, EAF, and LF, adapted from Ref. [26], Copyright (2015), with permission from Elsevier. |
2.2 Scrap recycling
Steel scrap in steelmaking is typically used either in an EAF for scrap-based steelmaking or in a BOF for ore-based steelmaking. In BOF steelmaking, approximately 75% of the iron input is derived from hot metal produced via the BF, with the remaining 25% consisting of steel scrap [50]. In contrast, the EAF process typically utilizes up to 100% steel scrap, though a portion of direct reduced iron (DRI) can also be used.
Steel scrap can generally be classified into three major streams, namely home, prompt, and obsolete [51]. As can be seen in Figure 3 [52], firstly, home scrap is typically well-sorted by quality and contains low levels of impurities, making it suitable for recycling into the same steel grade. Secondly, prompt scrap comes from consumer goods manufacturing and is often well-sorted and of high purity. Lastly, obsolete scrap is generally classified into different categories based on its composition and size.
Impurities in the scrap mixture can be non-steel materials, i.e., plastic and copper fragments that were not separated after shredding, alloys within the steel scrap, and coatings increasingly used on steel products. Certain impurities can be eliminated as dust, generated from elements in coatings that vaporize before the scrap is melted and then settled out from the gas upon cooling. Other impurities oxidize to form metal oxides upon smelting [52–54]. The impurities that occur from scrap (phosphorus, sulfur, and tramp elements) can adversely influence steel properties [55].
Adopting technologies to clean scrap before it reaches steel furnaces is essential to maximizing the potential of low-quality scrap streams. In this regard, Björkman et al. [52] highlighted measures to minimize the detrimental effects of tramp elements in scrap, including design for recycling, improved sorting at the shredder plant, improved processing at the steel plant, and development of alloys that can accommodate impurity and tramp elements, diluting scrap with ore-based iron units.
Several approaches have been developed and used in steelmaking for optimizing scrap tracking and charge [56,57]. Tenova and ORI Martin, as part of the Lighthouse Steel 4.0 project [58], which received funding support from the Italian Ministry of Economic Development (MISE) and Region Lombardia, improved the classification and tracking process for scrap material from when it enters the steel plant to the moment it exits the EAF as molten steel. At ORI Martin, the scrap acceptance process involves weighing trucks, checking for radiation, and photographing the truck and its load. Tenova developed a system based on machine learning, using convolutional neural networks, to classify the scrap category automatically from the images. The scrap is then stored in the yard, with crane movements tracked along with information about the scrap type and supplier. A 3D laser scanner on the cranes profiles the scrap volumes, assisting in loading and unloading operations and creating a real-time 3D map of the yard. This information is used in the Consteel continuous charging system, which feeds and pre-heats materials to the EAF. Another camera system on the conveyor ensures that material that could damage electrodes does not enter the EAF uncontrolled. In addition, the correlation between scrap grade in the charge and the steel analysis at the end of each heat allows any discrepancy to be identified with respect to the expected composition (with particular attention to the tramp elements) and identify the scrap quality concerning the supplier.
Within the “Circularity enhancements by low-quality scrap analysis and refinement” (CAESAR HEU) project [59], brings together steelmakers, technology developers, and research centers to enhance the circularity of low-quality scrap. Advanced technologies will be developed to enhance scrap upgrading, sorting, and characterization to refine low-quality scrap streams within Europe. The project aims to identify opportunities to use and reuse lower-quality scrap through advanced characterization and sorting technologies, support high-quality steel production in the EAF and increase the scrap rate in the converter, and develop and implement an industrial demonstrator for scrap sorting/cleaning using innovative technologies [60].
However, the analysis conducted by Oda et al. [61] indicated that the global availability of scrap would be limited. The study emphasized the critical importance of researching and developing innovative CO2-lean technologies for primary steelmaking, as well as assessing their economic feasibility.
 |
Fig. 3 Schematic representation of scrap recycling, adapted from Ref. [52], Copyright (2014), with permission from Elsevier. |
2.3 Recycling of biomass residues in iron and steelmaking
Traditionally, the primary methods for steel production include using fossil fuels [62,63], namely coal and natural gas; fuel, and reducing agents, namely coke [64] or carbon source (in addition to those mentioned, graphite and calcined petroleum coke can also be considered). As the world shifts towards environmentally conscious practices, integrating lignocellulosic biomass, biochar, or biocoke into iron and steelmaking processes has emerged as a promising path to reduce reliance on fossil fuels [65,66].
Prior to considering the possibility of the application of alternative carbon biomaterials (biomass, biochar, biocoke) in iron and steelmaking, it should be highlighted that raw biomass has several disadvantages, such as high moisture content, low calorific value, low bulk density, and high content of oxygenated volatile matters (VM). Therefore, in most cases, pre-treatment is inevitable to obtain bio-substitutes with properties allowing a partial or complete replacement of fossil fuels in metallurgical processes [34,35,67–70].
To mitigate these disadvantages, making pellets or briquettes is the simplest way to improve the physical and mechanical properties of the raw biomass [71–73]. Moreover, the composition of the compacted products can be easily adjusted to meet changing requirements and raw material availability. Additionally, improving the properties of raw biomass in obtaining biochar can be achieved via thermal treatment (e.g., torrefaction [74], hydrothermal processing [75–77], and pyrolysis [78,79], etc.). The advantages of thermal treatment include reduced moisture and oxygen content while increasing the carbon content and the calorific value of the solid product, making it more suitable for use in various metallurgical processes.
Regarding the torrefaction process, it is worth paying attention to the EU-funded project entitled “Converting wood waste into biofuel from steelmaking,” Torero [80] the aim of which is to introduce a novel concept of utilizing waste wood products that cannot be recycled and would otherwise be incinerated. The technology, developed and adapted by consortium partner TorrCoal, relies on torrefaction, a thermochemical process at high temperatures (250−320°C) without oxygen, thereby removing water and volatile matter from biomass [81]. The innovation behind Torero is that the biocoal generated can be utilized to replace the fossil fuels within the BF in steelmaking industrial installations. The waste wood is collected, appropriately processed, and subjected to torrefaction before it is transported to the BF instead of fossil coal. In collaboration with the STEELANOL project (Production of sustainable, advanced bio-ethANOL through an innovative gas-fermentation process using exhaust gases emitted in the STEEL industry) [82], the waste gas emissions from the steelmaking plant can then be converted into ethanol using a microbe-based fermentation process. Waste wood, in essence, generates a competitive input feedstock to produce renewable fuel, thus creating an additional value chain in the transport sector. Both projects successfully started their industrial production at the end of 2023; the plants are located close to the ArcelorMittal operations in Ghent, Belgium (Fig. 4).
It should be mentioned that utilizing alternative carbon biomaterials in metallurgical processes is not a new concept; it is a well-established, sustained practice that has been extensively outlined and reported. There is a practice of using alternative carbon biomaterial in various metallurgical processes: for the carbonization (cokemaking) process [83–86], in BF: as a charged fuel and reducing agent [67,87], and an injected one [88,89], in iron ore sintering [90,91], in EAF [92,93], and in the direct reduction of iron [94–96], etc.
 |
Fig. 4 Steelanol (a); and Torero (b) demonstration plants. |
2.3.1 Utilization in cokemaking
Conventional coke is primarily consumed in BF production, where specific quality requirements are crucial for its practical use. Coke serves several essential functions in BF ironmaking. Firstly, it maintains the burden structure and sustains skeleton permeability and drainage efficiency. Secondly, it acts as a reducing agent, providing chemical energy for melting the burden. Lastly, coke contributes carbon to the carburization of hot metal and acts as a filter for soot and dust [97]. While the first and last functions are indispensable in BF, the other roles are more flexible and subject to potential alternatives.
It should also consider the critical fuel and reducing agents parameters: the coke reactivity index (CRI) and coke strength after the reaction with CO2 (CSR). European BF requirements dictate a CRI of 23% and a CSR of 65%. Additionally, good mechanical strength is essential, under the Micum test: M40 (>88%), M25 (>90%), and M10 (<6%) representing the percentage of material grain sizes remaining above 40 mm, 25 mm, and 10 mm, respectively, after a mechanical treatment (100 revolutions in a drum). From the IRSID test: I40 (>57%), and I10 (<18%) represent grain sizes of +40 mm and −10 mm in % (500 revolutions in a drum) [98]. This strict tolerance for fuel quality and reducing agents is a limiting factor for BF applications of alternative carbon biomaterials.
Due to the challenges posed by the low bulk density, low strength, and difficulty maintaining the required particle size of biomass or biochar, the potential utilization of them as fuel and reducing agents is a complex technological task. In this context, the production of biocoke emerges as a viable consideration for BF applications.
Nevertheless, biomass, particularly charcoal, has been historically used in cokemaking, with evidence suggesting that charcoal, derived from wood or other organic materials, was employed in early cokemaking processes. Using biomass/biochar in the coal blend to obtain biocoke has several advantages: some of them are obvious: the benefit of a CO2-neutral carbon source and lower sulfur and phosphorus content than conventional coke derived from only a coal blend. Another one can be considered controversial, relating to increasing the reactivity of coke to decrease the temperature of the thermal reserve zone of the BF [98], thereby decreasing the amount of coke required to produce a ton of hot metal [98,99]. Nevertheless, according to [100], producing biocoke with specific physical and chemical properties remains challenging, primarily due to its lower CSR and higher CRI than conventional metallurgical coke. The addition of biomass to the coal blend in cokemaking is currently limited to a range of 2–10%, necessitating further research to optimize biomass utilization without compromising the quality of the resulting biocoke. Key parameters such as fixed carbon, volatile matter, alkali content, particle size, and biomass product reactivity must be carefully optimized before blending with coal.
Efficient production of high-quality biocoke has been demonstrated, with results comparable to coke obtained from a coal blend, as long as the biomass content does not exceed 10% [67]. This limitation also applies to pre-carbonized biomass [84].
Focusing on studies carried out regarding the influence of bio-additives on biocoke quality, research by MacPhee et al. [84] should be mentioned, which reveals a linear decrease in CSR and an increase in CRI when charcoal is added in increasing amounts from 2 to 10%.
Ng et al. [101] used charcoal additives in a coal blend to produce biocoke, observing similar CO2 reactivity to reference coke with 2% and 3% charcoal additions. In comparison, 5% charcoal resulted in lower initial gasification temperature and higher reaction kinetics. In another study [102], the authors used hydrochars obtained by hydrous pyrolysis from a pine Kraft lignin to produce biocoke. It was concluded that resulting biocokes are more reactive than coke made from good coking coal, and the micro-strength of biocokes is much lower than that of conventional coke.
In another study, the amount of biomass additives was considered up to 5% for two industrial coal blends [83], the types of biomass being chestnut and pine. It was concluded as the amount of biomass used increased, the CRI increased, and the CSR decreased. However, all indicators were within acceptable limits, and the authors also concluded that the amount of biomass added was low to prevent any deterioration in coke quality.
Moreover, Xing [103] produced coke with a 7.5 wt.% charcoal addition. The author studied the influences of charcoal addition on coke properties under gasification and annealing, simulating the conditions within an ironmaking BF. The charcoal did not significantly affect the coke pore structure during BF annealing. Gasification in the BF caused a greater change in the biocoke pore structure associated with charcoal addition due to the preferential consumption of charcoal particles by the Boudouard reaction.
In study [104], it was shown that by applying some treatment methods, such as briquetting and high pyrolysis temperature for lignin-based biocarbon, some important properties (mechanical strength, apparent density, and reactivity) could be improved. Moreover, the compressive strength property of lignin-based biocarbon can surpass the standard metallurgical coke. Additionally, lignin-based biocarbon can be considered for utilization in pyrometallurgical processes (BF, EAF and SAF) to substitute fossil-based carbon.
The project “BIOCode − Biomass for COkemaking Decarbonization”, aims to reduce the carbon footprint in industrial cokemaking. It focuses on replacing some fossil coal in coking blends with carbon-neutral biomass, showing promise in decarbonizing this industry [105]. It aims to study this substitution through experiments conducted across various scales, ranging from laboratory trials to industrial-scale testing. Embracing the principles of CE, the project emphasizes utilizing biomass sourced from the wood industry and agroforest residues (Fig. 5, [106]). This initiative extends beyond environmental goals, striving to establish local biomass recovery, storage, and pre-treatment networks. The project chooses biomass selectively and applies pre-treatments, aiming for an industrial substitution rate of up to 10%.
This will involve utilizing agricultural and forestry waste from nearby industrial areas. To maximize biomass incorporation into coal blends, pre-treatments like pyrolysis and torrefaction are promising techniques to improve biomass quality.
2.3.2 Utilization in iron ore sintering
Iron ore sintering occurs at temperatures of 1200–1400 °C, during which a mixture of iron ore fines and other materials (e.g., sinter return fines, limestone) is used [107]. In the iron ore sintering process, biomass can be effective in the reduction of CO2 emissions and more effective in the mitigation of SOx and NOx. The utilization of biochar, with relatively high fixed carbon (>90%) and somewhat larger size (1–5 mm), can achieve sinter yield and sinter productivity equal to that obtained by coke breeze. Using a coke-biochar composite can enhance the replacement ratio of coal by up to 60% [90,91,108–111].
Regarding the results of the study on the use of biomass, by [112], biomass products should have a hard structure and low VM that can be suitable for substituting coke breeze in the iron ore sintering process. As reported by Kawaguchi and Hara [110], to achieve the necessary productivity and an equivalent sinter yield compared to using coke breeze, the biofuel’s fixed carbon content should exceed 90%. Additionally, the particle size of the biofuel should ideally fall within the range of 1-5 mm.
According to Jahanshahi et al. [113], for sintering purposes, the ideal charcoal fuel should possess characteristics such as low VM (<3%), high density, low reactivity, and particle sizes between 0.3–3 mm.
Concerning the possible replacement of coke breeze, Ooi et al. [114] studied the sintering process with fuel containing different amounts of sunflower husk and coke. The fuel blends were made using coke and sunflower husk based on the calorific value of 5% coke breeze. The results showed that the replacement of 10% of coke with sunflower husk did not significantly change the characteristics of the sintering process or the sinter quality but contributed to the decrease in the formation of 2,3,7,8-PCDD/Fs by approximately 10% (from 1 to 0.91 ng/Nm3). In the case of replacing 20% of coke with charcoal, the emissions of dioxins decreased by approximately 33%.
Jha et al. [115] carried out the iron ore sintering, applying sawdust and charcoal as partial substitutes for coke breeze. The study determined that a blend incorporating 10% sawdust, 30% charcoal, and 30% through a combination of sawdust and charcoal is the most suitable substitution for coke. This conclusion was drawn based on the sinters’ observed strength, reducibility, thermal degradation, and reduction degradation indexes.
In [116], the impact of incremental charcoal content, ranging up to 100%, in fuel blends used for sintering was explored. The findings indicated that the ideal charcoal content for these blends was around 30%. The substitution of up to 30% of coke breeze with charcoal in the iron-ore sinter blend yielded several beneficial outcomes. This substitution increased the sintering process’s vertical velocity, resulting in higher yield, strength, and improved reducibility by hydrogen at 800°C in the final sintered product. At this 30% charcoal substitution level, the sintering machine exhibited optimal efficiency, reaching approximately 58.51%, while the productivity in a BF was about 46.11%.
Cheng et al. [117] conducted various sintering tests by altering the charcoal content in the blend (20%, 40%, 60%, 80%, and 100%) while keeping other parameters constant. The results highlighted a change in sintering characteristics, mainly when charcoal replacement rates were high. This change was attributed to the efficiency of combustion and heat recovery. As the substitute surpassed 60%, the duration of melting temperature and the melt quantity index notably reduced. Another significant finding was the decrease in NOx emissions concentration as the charcoal proportion increased in the sintering process.
2.3.3 Utilization in carbon composite agglomerates (CCAs)
CCAs are mainly used in the BF and the direct reduction (DR) process. Conventional CCAs are produced as pellets by cold bonding with or without a binder or by hot or cold pressing briquettes. The biomass used in the pellets can be raw biomass or charcoal. At a high temperature, iron ore can be reduced by biomass within the pellets via the following reactions [118–120]:
On the one hand, the reactivity requirements for carbon materials are not very stringent. Nevertheless, the carbon in the CCAs should not participate in any chemical reactions below the set temperature of the heat reserve zone. One of the most important quality parameters is the strength of the CCAs. Top-loaded CCAs should meet a BF’s minimum mechanical strength requirements. Otherwise, they can worsen the efficiency of the process. In addition, carbon-bearing biomaterials directly affect the mechanism of mass and heat transfer, temperature profile, and gas distribution inside the BF. Chemical reactions lead to the formation of gases within the pellet, increasing its porosity. As reported by Mousa et al. [121], the main disadvantage of CCAs is their low crushing strength. Up to 46% of biochar can be used in the DR process in agglomerates. It has been pointed out that the reduction rate of iron oxide is higher in biochar-based CCAs due to its greater reactivity than conventional coal or coke-based CCAs [122,123]. Further studies are needed on the production aspects of these pellets, especially since their use in a BF requires higher strength qualities.
2.3.4 Utilization in blast furnace
The main ways to use alternative carbon biomaterial in a BF are top charge (in this case, biocoke can be suitable) and pulverized injection (in this case, biochar or charcoal can be suitable). Charging alternative carbon biomaterial is problematic to achieve due to the minimum tolerance of the BF to the quality of coke or biocoke, which is justified by the fact that there are many factors in the BF (shattering, abrasion, solution loss reaction, alkaline attack, high-temperature attack and breakage by high-speed hot blast) [98] affecting the degradation of coke or biocoke.
In turn, utilizing torrefied biomass, charcoal, or biochar in the BF as pulverized fuel can change the conditions in the BF, influencing the coal and coke rate and quality requirements. Firstly, torrefied biomass, charcoal, or biochar injection into the BF has an effect on the coke reactivity and degradation mechanism. Moreover, there can be variations in the capacity of decreasing reducing agent rates and changes under in-furnace conditions depending on the physical and mechanical properties of the torrefied biomass, charcoal, or biochar.
In spite of this, Brazil is an example that produces hot metal on a large-scale using pulverized charcoal injection at a rate of 100-200 kg/tHM in a mini BF [124,125]. Currently, pulverized charcoal, or a mixture of pulverized charcoal and pulverized coal (PCI) in a mini BF (50–350 m3) can produce 10 Mt of steel. Maintaining efficient performance requires specific characteristics for charged materials (pellets, sinter, coke). Hot and cold burden strengths play a significant role in sustaining the permeability of the shaft. The advantages of the mini BF technology are low emissions, the low sulfur content in the iron, and low slag volumes.
Concerning the laboratory scale outcomes, Orre et al. [126] evaluated the performance in the case of using different biomass and visualized results via RIST- and carbon direct reduction rate (CDRR) diagrams. In this study, the injection of torrefied biomass or charcoal, top charcoal charging, and their combination were evaluated in model calculations. It was found that injecting 142 kg/tHM of torrefied biomass significantly impacted the BF conditions and could be counteracted by top-charging 30 kg/tHM of charcoal. With the combined use of the latter methods, CO2 emissions can be reduced by up to 34% with moderate changes under BF conditions and limited investments.
In turn, de Castro et al. [127] presented a six-phase mathematical model of the BF, which can simulate the BF operation under simultaneous PCI and charcoal. The model results indicated that a further decrease in coke consumption in the BF could be possible by using combined injections of 150 kg of pulverized coal and 100 kg/tHM of pulverized charcoal.
Wang et al. [128] used hydrothermal carbonization (HTC) technology to carbonize and improve biomass raw material to obtain hydrochar for BF injection purposes. The effects of HTC temperature and holding time on the yield, composition, structure, combustion behavior, and safety of hydrochar were studied. Since biomass hydrochar has the characteristic of being carbon neutral, BF injection hydrochar can reduce CO2 emissions, and every 1 kg/tHM of biomass hydrochar can reduce CO2 emissions by 1.95 kg/tHM.
Sundqvist Ökvist et al. [129] evaluated the introduction of biocoal into the BF via biocoke, biobriquettes, and biocoal injection and concluded that these addition methods are possible. Industrial results verify that injection of up to 10% of biocoal mixed with coal or the addition of some percentages of biocoal to residue briquettes can be applied in the short term and reduce the fossil CO2 emissions if enough biocoal is available.
According to Suopajärvi et al. [130], the replacement ratio of fossil-based reducing agents achieved with charcoal is the highest, thus leading to the largest decrease in fossil-based coke consumption. The amount of charcoal injected into the BF per produced ton of hot metal could be around 200 kg, implying a coke rate of 260 kg/tHM. Charcoal production technologies can be kept rather uncomplicated, and integration with heat and power applications is straightforward. On the other hand, the yield of charcoal from slow pyrolysis is low, and much of the chemical energy of biomass goes to pyrolysis liquids and permanent gases. Fast pyrolysis bio-oil does not have suitable properties for BF injection but can be used in heat and power production as such and, with further upgrading, in several other applications. The by-product char (15–25 wt.%) from fast pyrolysis could also be utilized in reducing agent applications. Solid and liquid bio-based reducing agents can be produced in a decentralized manner, whereas gaseous bio-reducers require a centralized production scheme [130]. In summary, from the ironmaking perspective (Fig. 6), the most promising bioreducing agent scenario could involve using charcoal in the BF.
 |
Fig. 6 Pathways to produce reducing agents for the metallurgical industry from biomass by thermochemical conversion, reproduced from Ref. [130], Copyright (2013), with permission from Elsevier. |
2.3.5 Utilization in electric arc furnace
EAF primarily uses electricity with a small amount of carbon material [131]. This carbon material serves various functions in the EAF process, including providing chemical energy, creating a reducing atmosphere during smelting to minimize oxidation, acting as a carbon source for slag foaming [132,133] to enhance energy efficiency and productivity.
Several different conclusions have been made regarding using biomass or biochar as an individual carbon source or mixtures of biochar and coke [134–140]. In papers [134–137], promising results were obtained suggesting potential applications in slag foaming or cogeneration of plants in the scrap-EAF route. Moreover, Yunos et al. [136] explored the use of palm shell char in the EAF, showing improved interaction with EAF slag compared to conventional coke. Additionally, Fidalgo et al. [137] studied biochars from agricultural residues, such as grape seed and pumpkin seed chars, as potential replacements for coal in EAF steelmaking. They found that biochar could effectively replace coal, and its high VM was conducive to slag foaming. Some studies [132,138] have indicated that employing a blend of coke and biochar (for instance, 50 wt.%:50 wt.% or 70 wt.%:30 wt.%) can enhance slag foaming or obtain superior outcomes compared to using biochar individually. It was concluded [138] that the reaction of the nut coke primarily caused the slag foaming, while the role of the loose biochar remained uncertain.
However, it has also been reported that results with the application of biochar individually can be poorer than conventional sources [132,138]. To be more precise, Huang et al. [139] investigated the interaction between synthetic slag and carbon materials from various sources, including biochar, and observed that biochar had a weaker interaction with slag than other carbonaceous materials, potentially affecting slag foaming. In turn, Funke et al. [140] explored the application of char from wheat straw fast pyrolysis as charge carbon in an EAF. However, the presence of minerals, especially potassium, posed challenges, requiring optimization for EAF use.
Within the “Sustainable EAF steel production” project, GreenEAF [141] studies were carried out on the partial or full replacement of coal and natural gas with charcoal or syngas produced from biomass pyrolysis in EAF steel production. Charcoal showed poor foaming results due to differences in wettability, affecting the interaction with slag; the volatile matter content of carbon sources did not significantly influence slag foaming in these tests.
Cirilli et al. [142] conducted tests for replacing anthracite with biochar. The results of the industrial tests indicate that char utilization as charge material can be done, but operating practice needs to be optimized with long-term experimentation. As part of the following RFCS project, (Biochar for a sustainable EAF steel production, GreenEAF2) [143], industrial trials demonstrated the feasibility of using biochar as charge material without significant modifications to the steel and slag analysis.
At the Swedish metals research institute Swerim [144], three types of hydrochars (a form of biocoal made through hydrothermal carbonization) derived from orange peel, green waste, and rice husk were tested in a 10-ton test-bed EAF. These hydrochars were injected into the EAF and placed on top as a carburizer to replace anthracite [145]. Additionally, introducing hydrochar from the top into the EAF at the start of the process led to a higher yield of carburization compared to the injected one. The yield of fixed carbon to steel was higher for hydrochar than for anthracite. However, the yield of Ctot was lower for hydrochar due to the higher VM. The final levels of phosphorus and sulfur in the liquid steel, with the addition of hydrochars, were maintained within acceptable limits.
Under another initiative, the RFCS BioReSteel project “Valorization of wet biomass residues for sustainable steel production with efficient nutrient recycling” [145] attempts to replace fossil carbon in the EAF by biocoal, produced from low-value locally available wet biomass residues by means of a hydrothermal carbonization process. An experimental study will prove the concept by employing laboratory and EAF testbed trials. The industrial EAF trials will be performed at three EAF plants to test hydrochar injection, hydrochar top charging and bio-oxide agglomerates to prove the concept’s flexibility and generality.
Simulation tools can be useful to develop scenario analyses that can drive and extend the outcomes of field trials in the context of the use of biomass and biochar in the EAF, such as those shown by the recent Horizon Europe project entitled “Gradual integration of REnewable non-fossil ENergy sources and modular HEATing technologies in EAF for progressive CO2 decrease” (GreenHeatEAF). Here an Aspen Plus-based model of the EAF process is adopted to develop sensitivity analyses related to the main parameters (e.g., moisture, сarbon and sulfur content) of biochar added in the EAF process with respect to steel and slag compositions and process performances in terms, of energy, metallic efficiency and CO2 emissions, for instance.
It is worth noting that although biomass has several advantages, such as those mentioned above, along with its use in iron and steelmaking, it is burdened by several challenges associated primarily with its properties and its impact on the further process in which it can be used. Additionally, the limited availability of sustainably producible biomass is a critical concern. The competition for land and resources between biomass production, food agriculture, and biodiversity preservation limits the amount of biomass that can be harvested ethically and sustainably. Balancing the use of lignocellulosic biomass with the need for environmental conservation requires careful planning and effective management strategies. The widespread use of biomass in iron and steel production could significantly increase the demand for woody biomass, raising concerns about deforestation. Moreover, biomass availability within the EU is becoming a pressing issue due to rising demand from other sectors, such as pulp and paper, natural fibers, chemicals, and the heating and transport industries [100]. Additionally, the development and use of biomass in iron and steel production rely heavily on key factors such as the availability of sustainable domestic biomass resources, supply chain logistics, and supportive national policies. Concerning the above-mentioned, Mandova et al. [146] identified several opportunities for integrating domestically sourced biomass into blast furnace ironmaking. Countries like Canada, Sweden, China, the USA, and France were highlighted as the most suitable due to favorable conditions. However, other nations may encounter challenges such as limited government support or inadequate biomass resources.
2.4 Recycling of polymers in iron and steelmaking
Polymers utilization has shown the potential to mitigate known environmental issues [147] even if they are not considered renewable carbon sources. The last decade has seen a 50% growth in plastics production, which is expected to continue in the coming years [22]. The chemical composition and physical characteristics of waste plastics play a crucial role in determining their suitability for reduction processes. Plastics typically contain lower sulfur and alkaline amounts than traditional fuels like coal, heavy oil, or coke [148].
Despite the abundance of discarded plastic materials, the recycling industry faces challenges in managing certain plastics, especially mixed and contaminated materials. Mechanical separation encounters limitations due to sorting requirements and the deteriorating quality of materials with each recycling cycle.
Alternative polymer materials with the proper H2/C ratio (such as plastics or rubber from waste residue) to partially replace virgin fossil carbon sources in iron and steelmaking processes represent a potential for their utilization. Waste plastics can be utilized in ironmaking, namely cokemaking, and BF; or EAF steelmaking. Literature reports that approximately 30% of coke and coal can be replaced in EAF steelmaking by polyethylene (PE) wastes, resulting in energy savings of around 12 kWh/t of plastic charge. Moreover, substituting 1.7 kg of tires for 1 kg of anthracite in the steelmaking process contributes to lower CO2 emissions, particularly when using carbon-neutral natural rubber in tires [149].
2.4.1 Utilization in cokemaking and blast furnace
Using plastic in cokemaking faces two main limitations: the quality of the resulting coke should meet the specific requirements mentioned earlier in Section 2.3, and adding plastic significantly impacts coking pressure, potentially endangering coke ovens. Similar to considering biomass, determining the feasible amount of plastic for cokemaking is crucial in understanding its utilization. Regarding the impact of plastic addition on the resulting coke quality and coking process, extensive research has been carried out by Nomura et al. [150], who investigated chlorine behavior during the co-carbonization of coal and chloride compounds like polyvinyl chloride (PVC) in both laboratory-scale and commercial-scale coke ovens. In both tests, adding PVC to coal showed minimal impact on the chlorine content in coke and coke oven gas (COG). The chlorine residue ratio in coke from PVC was significantly lower than that from coal, which is attributed to the faster decomposition of PVC during carbonization compared to the chlorine release from coal. Moreover, Nomura et al. [151] studied the effect of plastic addition on coal caking properties. They found that thermal decomposition products of plastics interacted with bituminous coal during carbonization in coke ovens. The impact varied with the type of plastics; aliphatic polymers had a modest effect on coal caking and coke strength. In contrast, aromatic polymers decreased fluidity and dilatation, and worsened coke strength. In another study, Nomura [152] proposed the waste plastic recycling process in coke ovens as superior, capable of treating a large amount of waste plastics, recovering useful materials using existing facilities, maintaining coke quality, and fixing released chlorine in the ammonia liquor spray system. Eventually, Nomura and Kato [153] recycled waste plastics using coke ovens, co-carbonizing coals, and added plastics into coke, tar, oil, and coke oven gas. They investigated the effect of plastic particle size on coke quality and coking pressure, concluding that either large or small plastic particles are favorable for waste plastic addition without affecting coke strength. It was supposed that a 1% addition of large-size agglomerated waste plastics did not increase coking pressure. Notably, in [154], it was concluded that the addition rate of waste plastics to blended coals should be limited to 1 wt.% to avoid negatively affecting coke strength.
Concerning the application in BF, it is known that waste plastic injection via tuyeres, ranging from 60 to 80 kg/tHM, has been implemented in various countries [155]. These plastics exhibit lower thermal ignition temperatures, quicker burning times, faster-burning rates, reduced ash ratios, and higher calorific values than pulverized coal. The physical properties of waste plastic, such as porosity, shape, and size, impact reaction kinetics. The highest possible amount of waste plastic that can be injected is around 70 kg/tHM. Interestingly, one ton of plastics can substitute roughly 750 kg of coke [156]. According to Devasahayam [157], who investigated the addition of 2 wt.% waste plastics to coal blend in cokemaking, concluded that a 2% mitigation of BF CO2 emissions is possible. Plastic replacement in BF leads to energy resource savings totaling up to 60 GJ/t. Efficient resource use has enabled up to a 40% replacement of metallurgical coke with injected waste plastics.
From practical perspective, ArcelorMittal Belgium is partnering with Vanheede Environment Group, Ghent University, and CRM Group on the “SMART: Steelmaking with Alternative Reductants” project [158]. It aims to reduce CO2 emissions by chemically recycling end-of-life plastics and other waste in the steelmaking process. This initiative aligns with ArcelorMittal Belgium’s sustainability strategy to lower CO2 emissions by 35% by 2030 (compared to 2018) and move toward climate neutrality by 2050, which aligns with the Green Deal framework. The SMART project focuses on substituting traditional fossil-based carbon reductants like coal with not-yet recyclable waste-based alternatives.
2.4.2 Utilization in electric arc furnace
Using polymers from plastic waste in EAF steelmaking offers advantages both in terms of energy supply and as a carbon source.
If the focus is shifted to laboratory-scale research, rubber-derived carbon can show high reactivity and a low reduction rate of iron (II) oxide from slag, according to an investigation by Maroufi et al. [159]. The significant role of carbon material and slag interaction in the iron oxide reduction process was emphasized. Contrary to this conclusion, when using coke/plastics (HDPE), the FeO reduction rate in slags with high FeO content is significant compared to coke. Moreover, the CO2 content in the off-gas was observed to decrease (by 75%) with an increase in the polymer content of the blend [15].
Zaharia et al. [160] explored the effect of adding waste rubber tires to blends with coke for carbon injection in EAF steelmaking. This study suggests that higher combustion efficiencies can be achieved when coke is partially replaced with waste rubber. At an industrial level, the simultaneous injection of coke and rubber in EAF steelmaking under an oxygen and nitrogen atmosphere is expected to somewhat enhance furnace efficiency.
Several studies have explored the application of polymers from waste plastics and rubbers in industrial EAF steelmaking processes [160–162]. In this light, the RFCS project OnlyPlastic (EAF working with polymers derived from plastic residue in substitution of fossil fuel, Project ID 899415) [163] substitutes all the fossil carbon sources (coal, coke, petroleum coke) in the Feralpi Lonato EAF, both injected and charged as reducing and foaming agents, with granulated densified polymers derived from plastic residue in agreement with the UNI 10667-17 specifications. The design of a new Tenova wall-mounted injector has shown good efficiency in all phases of the EAF process for both densified polymers alone and together with lime. A life cycle assessment (LCA) study performed by Rina-CSM highlights the environmental benefits of using SRA in steel production: the reduction in Scope 1 CO2 emissions up to 30% (climate change); the reduction in landfilling of hard-to-recycle plastic materials (land use); and no impact on the air quality (particulate matter, ozone depletion, acidification).
To summarize, when considering the use of polymers from plastic waste in metallurgical processes, even a small substitution is advantageous in the case of application in cokemaking and BF. Practically, utilizing plastics currently addresses only a small portion of end-of-life plastic disposal in the short term. However, it is crucial to recognize that integrating waste plastics in cokemaking is not a comprehensive solution for achieving environmental mitigation issues in coke production. From another perspective, in EAF steelmaking, using a mixture of coke and polymers is more favorable for slag foaming and reducing the iron oxide in the slag than using coke individually as a carbon source. This beneficial effect can also be noticed when using coke with biochar as a mix, as pointed out in Section 2.3.
3 Circularity of non-steel resources and industrial symbiosis
One of the main pillars of a CE is the closure of material cycles. Regarding the steel sector, several material cycles are evident apart from the steel cycle (i.e., recycling of scrap and ferrous material fractions from iron and steelmaking by-products). The three main cycles are CO2, process gases, and wastewater. The exchange of materials and energy between energy and resource-intensive sectors is defined as IS (sector coupling), and the steel sector can play a central role in such approaches.
3.1 Closure of the carbon cycle as a possibility for industrial symbiosis
To reach climate neutrality, carbon direct avoidance (CDA) must be the favored decarbonization pathway [164]. However, in a future climate-neutral steel sector, a certain amount of CO2 will still be released from the processes, even if secondary carbon carriers replace fossil carbon. Exemplarily, CO2 will be generated during the EAF process, where carbon is used for final iron oxide reduction or to create a foamy slag. In the lime producing sector, also important for steelmaking, or in cement production, CO2 is released during limestone calcination or the cement clinker process. These CO2 streams represent valuable raw materials, and carbon cycles can be closed by CCUS applications.
Known technologies to separate CO2 from process gases are amine scrubbing with different solvents [165] or membrane separation. Different approaches exist linked to the steel sector, in which CO2 is converted into valuable products such as methane [164] or ethanol [166]. The Austrian-funded project called “ZEUS” (Zero emissions through sector coupling) is an example of the steel and cement sectors being coupled. An amine scrubbing plant is installed at the pilot-scale level in a steel plant environment. ∼800 kg CO2 is captured daily from a power plant off-gas fired by steel mill process gas streams with a CO2 content between 20 and 25 vol.% [164]. The CO2 captured is compressed and filled into bottles and used in a catalytic methanation plant, which is also installed. This plant uses green H2 from an existing electrolysis plant to produce synthetic methane, which can be transported in existing natural gas pipelines and used in the steel plant as an energy carrier or reducing agent. In the scope of ZEUS, CO2 from the cement clinker process gas is separated by a membrane-based system and further used in an electrochemical synthesis to produce syngas or formic acid (see Fig. 7). By converting the CO2 into synthetic methane for reuse in the steel or cement industry, a carbon cycle is realized since the CO2 generated from methane combustion can be again separated and reused. Alternatively, chemical products can be used in the chemical industry. Both measures can contribute to the approach to climate neutrality. The project’s goal is to demonstrate sector coupling mechanisms by CO2-neutral process chains in the industrial environment (TRL>6) and develop business cases (efficiency vs. cost analysis).
 |
Fig. 7 Austrian-funded ZEUS project to demonstrate a cross-sectoral climate-neutral carbon cycle for accelerating the technology transfer into practice, reproduced from Ref. [164]. |
3.2 Enhanced water circularity in steel mills
Water is an important and special resource for iron and steelmaking, used for many purposes, such as cooling operations, descaling, or dust scrubbing. All types of water are used in steelmaking processes. Fresh water is mainly used in processes for direct and indirect cooling, while seawater is normally used for once-through cooling after an antifouling pretreatment. The average water intake for integrated steelworks is 28.6 m3 per ton of produced steel, with an average water discharge of 25.3 m3/ton of steel. For the EAF route, the average intake is 28.1 m3 per ton of steel, with an average discharge of 26.5 m3 per ton of steel [167]. Water has, therefore, a special status in the steelmaking process chain, and an efficient use of water resources is one of the main challenges of the steel sector, according to the EU water policy. In general, the main factors regarding industrial water performance are physical (flow velocity, temperature, pressure, etc.), chemical (pH, total dissolved solids, organic matter, metallic ions, etc.), and biological factors (aerobic and anaerobic bacteria). Therefore, process waters from steelworks and their treatment optimization need to be studied to decrease or eliminate pollutants originating from different steelmaking processes for improved internal water reuse. A research project funded under RFCS (Research Fund for Coal and Steel, RFCS) called “WHAM” (Water and related energy hub advanced management system in steelworks) focused, among others, on the minimization of cooling water losses and water management inefficiencies or increased water reusability through using innovative water treatments. Apart from that, sensors and accompanying models have been developed and tested as DSS for different applications. Exemplarily, a DSS for the optimal management of the rolling mill indirect cooling water system was tested in offline mode (see Fig. 8, [168]). In Figure 8, the pink point-line square underlines the boundaries of the case study; the light blue circles represent the well; the blue blocks are the basins; yellow, violet, and green blocks are the oxygen production plant, reverse osmosis, and cooling towers, respectively; and the orange block indicates the user process (wire rod mill). The make-up water sources are highlighted through dashed red squares.
Tests revealed that saving ∼4% and 95% osmotic and well water and increased use of low-quality water was possible [168]. The DSS was installed at an Italian steel producer’s site and is currently being tested in online mode.
 |
Fig. 8 A simplified flowsheet of the water circuit considered in the rolling mill use case, reproduced from Ref. [168]. |
3.3 Skills for industrial symbiosis
IS is characterized as a transaction of residual materials, water, and energy of a production process as inputs of other processes within the same company or among different companies [169]. The energy-intensive industries employ people with many different skills and diverse knowledge, who can work in multi-disciplinary teams. Steel mainly comprises the competence fields of metallurgy, materials science, physics, chemistry, engineering, environment, mathematics, information technology and computer science, languages, business, and accountancy [170]. The digital, green, and social transformation of the EU society includes a proactive industry-driven adjustment of the future skills demands of the different industrial sectors. Especially A.SPIRE, the SPIRE Association (SPIRE stands for sustainable process industry through resource and energy efficiency), a public private partnership, coordinates huge, collaborative R&D&I under the umbrella of Horizon Europe. A research initiative started in 2019 within the European ERASMUS+ program, is called “SPIRE-SAIS”- (skills alliance for IS − a cross-sectoral blueprint for a sustainable process industry SPIRE). It is based on the fact that IS will lead to new jobs and professions but lacks skill updates [169,170], and there is a need for attracting and training the new workforce across sector boundaries [171]. SPIRE-SAIS mainly includes the sectors chemicals, steel, engineering, non-ferrous metals, minerals, water, cement, and ceramics and provides educational modules and tools for greater awareness of the needs and opportunities provided by improved IS and energy efficiency. New skills, including green and digital skills, will also be identified for the practical implementation of IS in globally competitive industries. During the project, current professional profiles existing in the companies of the project partners have been identified and summarized in cross-sectoral generic job profiles (see Fig. 9).
Figure 9 clearly shows a strong connection between the various levels and sectors across different industries. Regarding production and functional areas, each area is represented by the related manager function (management level) and the dedicated operators/foremen/technicians (operational level). All these job profiles have internal company functions but could also become part of IS cooperation across sectors and companies. As it is evident that managerial skills and operational skills are different (at least concerning the concrete tasks and the level of skills), both management skills and operator skills are coming into focus. During improvement cycles, further pooling will be checked, e.g., to combine the Energy Manager, Environmental Manager, and Waste Manager in a common profile, “Environmental Engineering,” including those three job functions just as specific parts.
Regarding the steel sector addressed in SPIRE-SAIS and the European Steel Skills Alliance (ESSA), a simulation of the BOF or an interactive 3D model of the BF is implemented into the training platform, which is already a part of the ESSA online training platform steelHub. In the future, required skills will, among others, include aspects related to the limits and possibilities of electricity and hydrogen, recycling metals into usable metals and interaction between raw materials with strong quality fluctuations, LCA for process optimization, or the cleaning of water, gases, and slags [171]. This can be achieved by: a multidisciplinary approach, including green, digital skills and technical and transversal skills; a holistic approach for workforce reskilling and upskilling; a strong collaboration among industries, public bodies, and education providers.
 |
Fig. 9 Generic job profiles are generated by the sectors involved in the SPIRE-SAIS project (HR is Human Resources; OHS is Occupational Health and Safety), reproduced from Ref. [172]. |
4 Role of digitalization and digital solutions for recycling practices
The ongoing twin transition to a climate-neutral and digital economy is a challenge and an integrated approach, where digital tools can pave the way to a more efficient CE, i.e., they have the potential to enable an almost complete closure of material cycles. Due to the complexity of the digitalization process, the R&D&I directions must be addressed and supported to maintain and even increase actual trends and, looking to the defined entities, such as companies, make a forecast of relevant research activities and investments [173]. Using artificial intelligence (AI) and machine learning (ML) technologies can give greater support to operators by facilitating repetitive activities, improving steel product quality and quantity, improving the flow of information, reducing costs related to supply chain management, and supporting the improvement of workers’ health and safety conditions [174]. In the same manner, process simulation using tools such as LCA supports a deeper understanding of the environmental implications for metallurgical processes and the validation of solutions for enhanced energy and resource efficiency [171]. Similarly, the digitalization of environmentally-related information for the final product in a digital-product-passport (DPP) perspective introduces a potentially closer link between recycling rate and efficiency policy and related product claims to foreground users [175].
The role of digital solutions for recycling practices in the steel sector can be explained by scrap as one important secondary ferrous raw material. As explained in Section 2.2, scrap is used in both steelmaking furnaces, the BOF and the EAF, whereas in the BOF, scrap has the function of a cooling agent (temperature control due to exothermic oxidation reactions of silicon, manganese, and carbon from the hot metal) apart from its role as an iron carrier. In the EAF, scrap mainly serves as an iron carrier. Scrap trading according to its quality should be coupled with industrial transformation and circularity principles must be integrated into the steel business models. This means that different actions should be envisaged and linked to each other, and digital technologies (sensors and ML tools) play a key role [174]. By better controlling the melting processes, sensitivity to poor scrap qualities (e.g., “old” or post-consumer scrap) can possibly induce a higher reuse rate of post-consumer scrap. This is an important aspect since the volume of globally available post-consumer scrap is expected to increase to ∼900Mt by 2050 (compared to ∼400Mt post-consumer scrap currently available worldwide) [176]. Closely linked to the melting process, scrap yard logistics (either internally at the steel plant or an external scrap supplier in close cooperation with the steelmaker) is highly relevant.
Three main areas can be defined in which digital solutions can contribute to increased scrap recycling. First, at the scrap supplier site (before delivery to the steel plant), processing or purification techniques can be upgraded, for instance, by coupled optical and spectroscopic sensors integrated into the scrap processing line to improve the quality, for instance, of post-consumer scrap. Accompanied classification of different scrap types can be done by automated image analysis algorithms. Such optical and/or spectroscopic image acquisition is also possible directly on a truck or conveyor belt (at both, the scrap supplier site as well as later at the steel plant scrap yard). The second main area is the steel plant scrap yard, especially the selection of the different scrap grades as well as the transport to the melting furnace. Digital solutions can support optimized scrap yard management through automated selection and transport. Scrap is chosen according to the planned production sequences (steel product portfolio) based on the quality known. Monitoring of scrap charging linked to melting process performance represents the third area in which digital solutions can be applied. Scrap analysis before and during charging into the BOF/EAF with optical and spectroscopic methods coupled with AI techniques to identify scrap input analysis based on correlation with liquid steel analysis would be a possible scenario. The melting processes (energy demand, tap-to-tap time, additive usage, such as alloying elements or slag formers) are controlled more efficiently, inducing energy and resource savings.
Finally, in case all these aspects interact with each other satisfactorily, timely access to the scrap market enables the steel producer to acquire scrap qualities suitable for its production portfolio at the right time [174]. This leads to enhanced process and operation optimization and more efficient insights and outlooks on the scrap market.
5 Conclusions and outlook
The recycling trajectory in iron and steel production is promising but poses challenges. This review paper provides a comprehensive overview of research findings on recycling practices for scrap, iron and steelmaking slags, dusts, and process gases. It also highlights examples of ongoing and completed projects, underscoring the important role of recycling in achieving a cleaner future in the iron and steelmaking industry. It was revealed that integrating recycled materials, mainly scrap steel, is a critical step towards resource efficiency, allowing for significant reductions in energy consumption and CO2 emissions. However, recognizing the limited future availability of scrap worldwide requires precise research and innovative approaches to reduce emissions significantly. Efforts to manage and repurpose by-products such as slag, dust, off-gases, and water underscore the industry’s commitment to circularity and resource efficiency.
Incorporating alternative carbon biomaterials, namely biomass, biochar, or biocoke, into iron and steel production processes can open the way to reduce dependence on fossil fuel and reducing agents used conventionally, although with challenges on preconditions such as sufficient, sustainable domestic biomass resources, supply chain aspects, and supportive national policies. In addition, the requirements for the properties of fuel and reducing agents depend on the metallurgical process, which affects whether alternative carbon materials can be used as a partial replacement or completely.
Notable advances include using polymers from waste plastics in iron and steelmaking processes. However, polymers from waste plastics are not a renewable carbon source, and the possibility of their use is partial for some metallurgical processes, as discussed earlier. Additionally, it is important to understand that the use of plastics solves only part of the issue of recycling end-of-life plastics in the short term and is not a comprehensive solution to mitigate the environmental impact, which is instead based on a systemic vision for pursuing the efficiency of all processes involved.
While recycling remains vitally important, it alone cannot solve all challenges. Embracing CE principles, promoting the research and development of innovative technologies, and scaling up pilot projects can allow ambitious emissions reduction targets to be achieved. Going forward, a holistic approach integrating innovative technologies and CE principles, considering the opportunities for IS, along with digital solutions in recycling practices and related skills adjustments in time, will be critical to the transition of iron and steelmaking to more resource-efficient production processes.
Acknowledgments
The authors want to thank further colleagues contributing to the paper, which are Elizaveta Cheremisina, Irmela Kofler, Michael Derntl, and Wolfgang Reiter (K1-MET GmbH, Austria), Giorgio Porcu (Acciaierie d’Italia S.p.A., Italy), Bert Riems and Jeroen Van Lishout (ArcelorMittal Belgium NV), Jean-Christophe Pierret (CRM Group, Belgium), Costanzo Pietrosanti (Danieli Automation S.p.A., Italy), Klaus Peters and Delphine Snaet (European Steel Technology Platform ESTEP AISBL, Belgium), Jaume Pujante (Eurecat, Spain), David Algermissen (FEhS-Institut fuer Baustoffforschung, Germany), Anna Domenech (CELSA Group, Spain), Sanjeev Manocha (LanzaTech Inc., USA), Rita Kallio (University Oulu, Finland), Marco Simoni (former RINA-CSM, Italy) and Giovanni Bavastrelli as well as Mattia Bissoli (both from Tenova S.p.A., Italy). Comprehensive contributions from the content side originate from the ESTEP Annual Event entitled “A Circular Economy driven by European steel”, which was held in Barcelona (Spain) from October 3-5, 2023. The event was organized by ESTEP. Additionally, the authors appreciate the Reviewers for their thoughtful comments and suggestions, which allowed improving the quality of this paper.
Funding
The authors gratefully acknowledge the funding support of K1-MET GmbH, metallurgical competence center. The research program of the K1-MET competence center is supported by COMET (Competence Center for Excellent Technologies), the Austrian program for competence centers. COMET is funded by the Federal Ministry of Climate Action, Environment, Energy, Mobility, Innovation and Technology, the Federal Ministry of Labor and Economy, the Federal States of Upper Austria, Tyrol and Styria as well as the Styrian Business Promotion Agency (SFG) and the Standortagentur Tyrol. Furthermore, Upper Austrian Research GmbH continuously supports K1-MET. Additionally, I. Bellemans holds a grant from the Research Foundation Flanders (Research contract no. 1239024N). The authors are also grateful for the following projects: research conducted at the University of Oulu has been funded by Business Finland within two projects, which were TOCANEM (Grant Agreement no. 41700/31/2020) and FFS (Grant Agreement no. 45774/31/2020). Project iSlag received funding from the European Union within the RFCS (Grant Agreement no. 899164).Project ReMFra is funded by the European Union within the Horizon Europe Framework Program (Clean Steel Partnership, Grant Agreement no. 101058362). Project Lighthouse Steel 4.0 receives co-funding between the Italian Ministry of Economic Development (MISE) and Region Lombardia (Grant Agreement no. D.g.r. 19 dicembre 2018 - n. XI/1110). Project CAESAR is funded by the European Union within the Horizon Europe Framework Program (Clean Steel Partnership, Grant Agreement no 101058520). Project Torero received funding from the European Union’s Horizon 2020 Framework Program (Grant Agreement no. 745810). Project Steelanol received funding from the European Union’s Horizon 2020 Framework Program (Grant Agreement no. 656437). Additional funding was received from the Flemish program of Ecology and Transition Support, and the Belgian Energy Transition Fund. Project BIOCOde is funded by the European Union within the RFCS (Research Fund for Coal and Steel, Grant Agreement no. 101112264). Projects GREENEAF (Grant Agreement no. RFSR-CT-2009-00004) and GREENEAF2 (Grant Agreement no. RFSP-CT-2014-00003) both received funding from the European Union within the RFCS. Project BioReSteel is funded by the European Union within the RFCS (Grant Agreement no. 101112383). Project OSMET 3.0 is financially supported from the Swedish Governmental Agency for Innovation System (VINNOVA, Grant Agreement no. 2020-04140). Project GreenHeatEAF is funded by the European Union within the Horizon Europe Framework Program (Clean Steel Partnership, Grant Agreement no. 101092328). Project Smart-Life received funding from the European Union’s LIFE+ Program (LIFE19-CCM_BE_001215). Project OnlyPlastic received funding from the European Union within the RFCS (Grant Agreement no. 899415). Project ZEUS is funded by the Austrian Klima- und Energiefonds and is carried out within the Energy Research Program 2022 (FFG project number FO999903855). Project WHAM received funding from the European Union within the RFCS (Grant Agreement no. 800654). Project SPIRE-SAIS received funding from the European Union’s Erasmus+ Program (Grant Agreement no. 612429-EPP-1-2019-1-DE-EPPKA2-SSA-B). Project ESSA received funding from the European Union’s Erasmus+ Program (Grant Agreement no. 2018-3059 / 600886-EPP-1-2018-1-DE-EPPKA2-SSA-B).
Conflicts of interest
The authors declare no conflict of interest.
Data availability statement
The data presented in this study are available on request from the corresponding author.
Author contribution statement
Conceptualization, L.K. and J.R.; Writing − Original Draft Preparation, L.K. and J.R.; Writing − Review and Editing, L.K., J.R., R.A., A.S., W.S., H.O., E.P.H., G.D., C.M., D.M., L.D.S., F.C., V.C., T.B., I.M., A.P., A.Z., C.B., E.M., E.N., E.S., M.G., E.M., A.S., and I.B. All authors have read and agreed to the published version of the manuscript.
References
- G.-M. Kim, K.Y. Lisandy, Y.Y. Isworo, J.-H. Kim, C.-H. Jeon, Investigation into the effects of ash-free coal binder and torrefied biomass addition on coke strength and reactivity, Fuel 212, 487–497 (2018) [CrossRef] [Google Scholar]
- J. Madias, Electric furnace steelmaking, in: Treatise on Process Metallurgy (Elsevier, 2014), pp. 271–300 [CrossRef] [Google Scholar]
- J. Perpiñán, B. Peña, M. Bailera, V. Eveloy, P. Kannan, A. Raj, P. Lisbona, L.M. Romeo, Integration of carbon capture technologies in blast furnace based steel making: A comprehensive and systematic review, Fuel 336, 127074 (2023) [CrossRef] [Google Scholar]
- B. Rahmatmand, A. Tahmasebi, H. Lomas, T. Honeyands, P. Koshy, K. Hockings, A. Jayasekara, A technical review on coke rate and quality in low-carbon blast furnace ironmaking, Fuel 336, 127077 (2023) [CrossRef] [Google Scholar]
- J. Yu, R. Xu, J. Zhang, A. Zheng, A review on reduction technology of air pollutant in current China’s iron and steel industry, J. Cleaner Product. 414, 137659 (2023) [CrossRef] [Google Scholar]
- S. Cho, L. Tomas Da Rocha, B.-J. Chung, S.-M. Jung, Formation of NO and SO2 in the sintering process of iron ores, Metall. Mater. Trans. B 53, 84–95 (2022) [CrossRef] [Google Scholar]
- D. Reike, W.J.V. Vermeulen, S. Witjes, The circular economy: new or refurbished as CE 3.0? — Exploring controversies in the conceptualization of the circular economy through a focus on history and resource value retention options, Resour. Conserv. Recycl. 135, 246–264 (2018) [CrossRef] [Google Scholar]
- M. Rahnama Mobarakeh, T. Kienberger, Climate neutrality strategies for energy-intensive industries: an Austrian case study, Clean. Eng. Technol. 10, 100545 (2022) [CrossRef] [Google Scholar]
- R. Minunno, T. O’Grady, G.M. Morrison, R.L. Gruner, Investigating the embodied energy and carbon of buildings: a systematic literature review and meta-analysis of life cycle assessments, Renew. Sustain. Energy Rev. 143, 110935 (2021) [CrossRef] [Google Scholar]
- M. Östman, K. Lundkvist, M. Larsson, Environmental system effects when including scrap preheating and surface cleaning in steel making routes, in: Linköping, Sweden (2011) pp. 1684–1691 [Google Scholar]
- S. Biswal, F. Pahlevani, V. Sahajwalla, Wastes as resources in steelmaking industry — current trends, Curr. Opin. Green Sustain. Chem. 26, 100377 (2020) [CrossRef] [Google Scholar]
- H. Yi, G. Xu, H. Cheng, J. Wang, Y. Wan, H. Chen, An overview of utilization of steel slag, Proc. Environ Sci. 16, 791–801 (2012) [CrossRef] [Google Scholar]
- Slag recycling, (2020). https://www.recovery-worldwide.com/en/artikel/slag-recycling-3528047.html (accessed November 9, 2023). [Google Scholar]
- T. Norgate, N. Haque, M. Somerville, S. Jahanshahi, Biomass as a source of renewable carbon for iron and steelmaking, ISIJ Int. 52, 1472–1481 (2012) [CrossRef] [Google Scholar]
- J.R. Dankwah, P. Koshy, N.M. Saha-Chaudhury, P. O’Kane, C. Skidmore, D. Knights, V. Sahajwalla, Reduction of FeO in EAF steelmaking slag by metallurgical coke and waste plastics blends, ISIJ Int. 51, 498–507 (2011) [CrossRef] [Google Scholar]
- World Steel Association (2020). Water management in the steel industry. https://worldsteel.org/wp-content/uploads/Water-management-in-the-steel-industry.pdf (accessed August 19, 2024). [Google Scholar]
- Y. Meng, X. Zhu, Y. Zhang, Y. Su, F. Qu, C.S. Poon, J. Yan, D.C.W. Tsang, Valorizing inherent resources from waste streams for in-situ CO2 capture and sequestration in the steel industry, J. Clean. Prod. 458, 142486 (2024) [CrossRef] [Google Scholar]
- Y. Zheng, L. Gao, S. He, Analysis of the mechanism of energy consumption for CO2 capture in a power system, Energy 262, 125103 (2023) [CrossRef] [Google Scholar]
- L. Dong, H. Zhang, T. Fujita, S. Ohnishi, H. Li, M. Fujii, H. Dong, Environmental and economic gains of industrial symbiosis for Chinese iron/steel industry: Kawasaki’s experience and practice in Liuzhou and Jinan, J. Cleaner Prod. 59, 226–238 (2013) [CrossRef] [Google Scholar]
- M.T. Johansson, M. Söderström, Options for the Swedish steel industry − Energy efficiency measures and fuel conversion, Energy 36, 191–198 (2011) [CrossRef] [Google Scholar]
- E. da Costa, By-products and waste materials in steelmaking (1989). https://www.researchgate.net/publication/327633282_By-products_and_waste_materials_in_steelmaking. [Google Scholar]
- V. Colla, T.A. Branca, R. Pietruck, S. Wölfelschneider, A. Morillon, D. Algermissen, S. Rosendahl, H. Granbom, U. Martini, D. Snaet, Future research and developments on reuse and recycling of steelmaking by-products, Metals 13, 676 (2023) [CrossRef] [Google Scholar]
- J. Chen, Y. Xing, Y. Wang, W. Zhang, Z. Guo, W. Su, Application of iron and steel slags in mitigating greenhouse gas emissions: a review, Sci. Total Environ. 844, 157041 (2022) [CrossRef] [Google Scholar]
- H.T. Makkonen, J. Heino, L. Laitila, A. Hiltunen, E. Pöyliö, J. Härkki, Optimisation of steel plant recycling in Finland: dusts, scales and sludge, Resour. Conserv. Recycl. 35, 77–84 (2002) [CrossRef] [Google Scholar]
- A. Andersson, J. Isaksson, A. Lennartsson, F. Engström, Insights into the valorization of electric arc furnace slags as supplementary cementitious materials, J. Sustain. Metall. (2023) [Google Scholar]
- N.M. Piatak, M.B. Parsons, R.R. Seal, Characteristics and environmental aspects of slag: a review, Appl. Geochem. 57, 236–266 (2015) [CrossRef] [Google Scholar]
- Y.M. Yun, Y.S. Chu, S.K. Seo, J.H. Jeong, Analysis of reducing characteristics of direct reduced iron using blast furnace dust, J. Korean Ceram. Soc. 53, 444–449 (2016) [CrossRef] [Google Scholar]
- I. Cameron, M. Sukhram, K. Lefebvre, W. Davenport, The iron blast furnace process, in: Blast Furnace Ironmaking (Elsevier, 2020), pp. 1–18 [Google Scholar]
- J.M. Paris, J.G. Roessler, C.C. Ferraro, H.D. DeFord, T.G. Townsend, A review of waste products utilized as supplements to Portland cement in concrete, J. Cleaner Prod. 121, 1–18 (2016) [CrossRef] [Google Scholar]
- H. Shen, E. Forssberg, An overview of recovery of metals from slags, Waste Manag. 23, 933–949 (2003) [CrossRef] [Google Scholar]
- W. Johnson, The Effect of Chemical Composition of Blast-Furnace Slag on Compressive Strength and Durability Properties of Mortar Specimens (2017). https://digitalcommons.usf.edu/cgi/viewcontent.cgi?article=8607&context=etd ( accessed August 19, 2024). [Google Scholar]
- L.V. Fisher, A.R. Barron, The recycling and reuse of steelmaking slags — a review, Resour. Conserv. Recycl. 146, 244–255 (2019) [CrossRef] [Google Scholar]
- J. Rahou, H. Rezqi, M. El Ouahabi, N. Fagel, Characterization of Moroccan steel slag waste: The potential green resource for ceramic production, Constr. Build. Mater. 314, 125663 (2022) [CrossRef] [Google Scholar]
- R. Wei, L. Zhang, D. Cang, J. Li, X. Li, C.C. Xu, Current status and potential of biomass utilization in ferrous metallurgical industry, Renew. Sustain. Energy Rev. 68, 511–524 (2017) [CrossRef] [Google Scholar]
- S.V. Vassilev, D. Baxter, L.K. Andersen, C.G. Vassileva, T.J. Morgan, An overview of the organic and inorganic phase composition of biomass, Fuel 94, 1–33 (2012) [CrossRef] [Google Scholar]
- M. Guzzon, E.J. Chiarullo, M. Fulgosi, S. Porisiensi, Tenova’s dry granulation process. The experience of Pittini group on LF slag, in: Barcelona, Spain (2023) [Google Scholar]
- T.A. Branca, V. Colla, D. Algermissen, H. Granbom, U. Martini, A. Morillon, R. Pietruck, S. Rosendahl, Reuse and recycling of by-products in the steel sector: recent achievements paving the way to circular economy and industrial symbiosis in Europe, Metals 10, 345 (2020) [CrossRef] [Google Scholar]
- A. Petrucciani, A. Zaccara, I. Matino, V. Colla, M. Ferrer, Flowsheet model and simulation of produced slag in electric steelmaking to improve resource management and circular production, Chem. Eng. Trans. 96, 121–126 (2022) [Google Scholar]
- M. Falsafi, W. Terkaj, M. Guzzon, E. Malfa, R. Fornasiero, T. Tolio, Assessment of valorisation opportunities for secondary metallurgy slag through multi-criteria decision making, J. Cleaner Prod. 402, 136838 (2023) [CrossRef] [Google Scholar]
- I. Matino, V. Colla, T.A. Branca, L. Romaniello, Optimization of by-products reuse in the steel industry: valorization of secondary resources with a particular attention on their pelletization, Waste Biomass Valor 8, 2569–2581 (2017) [CrossRef] [Google Scholar]
- E. Nylund, Conditions for Industrial Symbiosis surrounding a hydrogen based steel industry, Master of Science Thesis, KTH Royal Institute of Technology (2023). https://www.diva-portal.org/smash/get/diva2:1772180/FULLTEXT01.pdf. [Google Scholar]
- J. Wang, Y. Zhang, K. Cui, T. Fu, J. Gao, S. Hussain, T.S. AlGarni, Pyrometallurgical recovery of zinc and valuable metals from electric arc furnace dust − a review, J. Cleaner Prod. 298, 126788 (2021) [CrossRef] [Google Scholar]
- M. Omran, T. Fabritius, Utilization of blast furnace sludge for the removal of zinc from steelmaking dusts using microwave heating, Separat. Purif. Technol. 210, 867–884 (2019) [CrossRef] [Google Scholar]
- Md. Anik Hasan, R. Hossain, V. Sahajwalla, Critical metals (Lithium and Zinc) recovery from battery waste, ores, brine, and steel dust: a review, Process Saf. Environ. Protect. 178, 976–994 (2023) [CrossRef] [Google Scholar]
- G. Pu, W. Du, H. Cheng, M. Tian, Z. Chen, Y. Chen, D. Ju, Research on biomass waste utilization for synergetic reduction of stainless steel sludge and zinc-containing dust, J. Sustain. Metall. (2023) [Google Scholar]
- M. Al-harahsheh, J. Al-Nu’airat, A. Al-Otoom, I. Al-hammouri, H. Al-jabali, M. Al-zoubi, S. Abu Al’asal, Treatments of electric arc furnace dust and halogenated plastic wastes: a review, J. Environ. Chem. Eng. 7, 102856 (2019) [CrossRef] [Google Scholar]
- REcovering Metals and Mineral FRAction from steelmaking residues, (2022). https://cordis.europa.eu/project/id/101058362 (accessed November 9, 2023). [Google Scholar]
- M. Simoni, W. Reiter, ReMFra − possibilities for residues from steel plants, in: Barcelona, Spain (2023) [Google Scholar]
- iRecovery System (2023). https://tenova.com/technologies/irecovery-system (accessed November 9, 2023). [Google Scholar]
- Y. Yang, K. Raipala, L. Holappa, Ironmaking, in: Treatise on Process Metallurgy (Elsevier, 2014), pp. 2–88 [CrossRef] [Google Scholar]
- V. Smil, Materials in Modern Iron and Steel Production, in: Still the Iron Age (Elsevier, 2016), pp. 115–138 [CrossRef] [Google Scholar]
- B. Björkman, C. Samuelsson, Recycling of steel, in: Handbook of Recycling, State-of-the-Art for Practitioners, Analysts, and Scientists ( Elsevier, Amsterdam, 2014) [Google Scholar]
- L.D.D. Harvey, Iron and steel recycling: review, conceptual model, irreducible mining requirements, and energy implications, Renew. Sustain. Energy Rev. 138, 110553 (2021) [CrossRef] [Google Scholar]
- L. Reijnders, Conserving functionality of relatively rare metals associated with steel life cycles: a review, J. Cleaner Prod. 131, 76–96 (2016) [CrossRef] [Google Scholar]
- T. Emi, Improving steelmaking and steel properties, in: Fundamentals of Metallurgy (Elsevier, 2005), pp. 503–554 [CrossRef] [Google Scholar]
- W. Wang, Cost optimization of scrap when making steel with an electric arc furnace, Master of Science Thesis, McGill University (2012) [Google Scholar]
- E. Sandberg, B. Lennox, P. Undvall, Scrap management by statistical evaluation of EAF process data, Control Eng. Pract. 15, 1063–1075 (2007) [CrossRef] [Google Scholar]
- Tenova and ORI Martin Launch their Lighthouse Plant “Acciaio_4.0 (2019). https://tenova.com/newsroom/press-releases/tenova-and-ori-martin-launch-their-lighthouse-plant-acciaio40 (accessed August 20, 2024) [Google Scholar]
- CirculArity Enhancements by Low quality Scrap Analysis and Refinement, CAESAR (2022). https://cordis.europa.eu/project/id/101058520 (accessed November 9, 2023) [Google Scholar]
- J.-C. Pierret, Circularity enhancements by low quality scrap analysis and refinement (CAESAR HEU project), in: Barcelona, Spain (2023) [Google Scholar]
- J. Oda, K. Akimoto, T. Tomoda, Long-term global availability of steel scrap, Resour. Conserv. Recycl. 81, 81–91 (2013) [CrossRef] [Google Scholar]
- L. Kieush, Coal pyrolysis products utilisation for synthesis of carbon nanotubes, Petrol. Coal 61, 461–463 (2019) [Google Scholar]
- L. Kieush, A. Koveria, J. Schenk, K. Rysbekov, V. Lozynskyi, H. Zheng, A. Matayev, Investigation into the effect of multi-component coal blends on properties of metallurgical coke via petrographic analysis under industrial conditions, Sustainability 14, 9947 (2022) [CrossRef] [Google Scholar]
- I. Cameron, M. Sukhram, K. Lefebvre, W. Davenport, Metallurgical coke − a key to blast furnace operations, in: Blast Furnace Ironmaking (Elsevier, 2020), pp. 557–572 [Google Scholar]
- L. Lu, J. Pan, D. Zhu, Alternative ironmaking processes and their ferrous burden quality requirements, in: Iron Ore (Elsevier, 2022), pp. 605–626 [CrossRef] [Google Scholar]
- C. Harpprecht, T. Naegler, B. Steubing, A. Tukker, S. Simon, Decarbonization scenarios for the iron and steel industry in context of a sectoral carbon budget: Germany as a case study, J. Cleaner Prod. 380, 134846 (2022) [CrossRef] [Google Scholar]
- H. Suopajärvi, K. Umeki, E. Mousa, A. Hedayati, H. Romar, A. Kemppainen, C. Wang, A. Phounglamcheik, S. Tuomikoski, N. Norberg, A. Andefors, M. Öhman, U. Lassi, T. Fabritius, Use of biomass in integrated steelmaking − Status quo, future needs and comparison to other low-CO2 steel production technologies, Appl. Energy 213, 384–407 (2018) [CrossRef] [Google Scholar]
- A. Demirbaş, Calculation of higher heating values of biomass fuels, Fuel 76, 431–434 (1997) [CrossRef] [Google Scholar]
- S.V. Vassilev, D. Baxter, L.K. Andersen, C.G. Vassileva, An overview of the chemical composition of biomass, Fuel 89, 913–933 (2010) [CrossRef] [Google Scholar]
- A. Pandey, S. Negi, P. Binod, C. Larroche, Handbook of pretreatment of biomass, processes and technologies (Elsevier, 2015) [Google Scholar]
- H. Rezaei, M. Tajilrou, J.S. Lee, K. Singaraveloo, A. Lau, S. Sokhansanj, Evolution of biomass particles during pelletization process, Particuology 86, 182–187 (2024) [CrossRef] [Google Scholar]
- Y. Li, High-pressure densification of wood residues to form an upgraded fuel, Biomass and Bioenergy 19, 177–186 (2000) [CrossRef] [Google Scholar]
- H. Rezaei, C.J. Lim, A. Lau, S. Sokhansanj, Size, shape and flow characterization of ground wood chip and ground wood pellet particles, Powder Technol. 301, 737–746 (2016) [CrossRef] [Google Scholar]
- K.B. Kota, S. Shenbagaraj, P.K. Sharma, A.K. Sharma, P.K. Ghodke, W.-H. Chen, Biomass torrefaction: an overview of process and technology assessment based on global readiness level, Fuel 324, 124663 (2022) [CrossRef] [Google Scholar]
- R. Wang, J. Jia, Q. Jin, H. Chen, H. Liu, Q. Yin, Z. Zhao, Forming mechanism of coke microparticles from polymerization of aqueous organics during hydrothermal carbonization process of biomass, Carbon 192, 50–60 (2022) [CrossRef] [Google Scholar]
- B. Babinszki, E. Jakab, Z. Sebestyén, M. Blazsó, B. Berényi, J. Kumar, B.B. Krishna, T. Bhaskar, Z. Czégény, Comparison of hydrothermal carbonization and torrefaction of azolla biomass: analysis of the solid products, J. Anal. Appl. Pyroly. 149, 104844 (2020) [CrossRef] [Google Scholar]
- B. Garcia, O. Alves, B. Rijo, G. Lourinho, C. Nobre, Biochar: production, applications, and market prospects in Portugal, Environments 9, 95 (2022) [CrossRef] [Google Scholar]
- X. Hu, M. Gholizadeh, Biomass pyrolysis: a review of the process development and challenges from initial researches up to the commercialisation stage, J. Energy Chem. 39, 109–143 (2019) [CrossRef] [Google Scholar]
- G. Wang, Y. Dai, H. Yang, Q. Xiong, K. Wang, J. Zhou, Y. Li, S. Wang, A review of recent advances in biomass pyrolysis, Energy Fuels 34, 15557–15578 (2020) [CrossRef] [Google Scholar]
- Converting wood waste into biofuel from steelmaking, Torero, (2017). https://cordis.europa.eu/article/id/443175-converting-wood-waste-into-biofuel-from-steelmaking (accessed November 13, 2023) [Google Scholar]
- B. Riems, G. Ounoughene, H. Draper, M. Hingsamer, I. Brenner-Fließer, G. Jungmeier, M. Hadler, The Torero project − substituting fossil carbon with biomass in steelmaking, in: Barcelona, Spain (2023) [Google Scholar]
- Production of sustainable, advanced bio-ethANOL through an innovative gas-fermentation process using exhaust gases emitted in the STEEL industry (2024). https://cordis.europa.eu/project/id/656437 (accessed November 14, 2023). [Google Scholar]
- M.G. Montiano, E. Díaz-Faes, C. Barriocanal, R. Alvarez, Influence of biomass on metallurgical coke quality, Fuel 116, 175–182 (2014) [CrossRef] [Google Scholar]
- J.A. MacPhee, J.F. Gransden, L. Giroux, J.T. Price, Possible CO2 mitigation via addition of charcoal to coking coal blends, Fuel Process. Technol. 90, 16–20 (2009) [CrossRef] [Google Scholar]
- M. Rejdak, R. Bigda, M. Wojtaszek, Use of alternative raw materials in coke-making: new insights in the use of lignites for blast furnace coke production, Energies 13, 2832 (2020) [CrossRef] [Google Scholar]
- T. Matsumura, M. Ichida, T. Nagasaka, K. Kato, Carbonization behaviour of woody biomass and resulting metallurgical coke properties, ISIJ Int. 48, 572–577 (2008) [CrossRef] [Google Scholar]
- T. Norgate, D. Langberg, Environmental and economic aspects of charcoal use in steelmaking, ISIJ Int. 49, 587–595 (2009) [CrossRef] [Google Scholar]
- A. Babich, D. Senk, M. Fernandez, Charcoal behaviour by its injection into the modern blast furnace, ISIJ Int. 50, 81–88 (2010) [CrossRef] [Google Scholar]
- J.A. De Castro, A.J. Da Silva, Y. Sasaki, J. Yagi, A six-phases 3-D model to study simultaneous injection of high rates of pulverized coal and charcoal into the blast furnace with oxygen enrichment, ISIJ Int. 51, 748–758 (2011) [CrossRef] [Google Scholar]
- G. Jha, S. Soren, Study on applicability of biomass in iron ore sintering process, Renew. Sustain. Energy Rev. 80, 399–407 (2017) [CrossRef] [Google Scholar]
- L. Kieush, M. Boyko, A. Koveria, A. Khudyakov, A. Ruban, Utilization of the prepyrolyzed technical hydrolysis lignin as a fuel for iron ore sintering, EEJET 1, 34–39 (2019) [CrossRef] [Google Scholar]
- R. Wei, X. Zheng, Y. Zhu, S. Feng, H. Long, C.C. Xu, Hydrothermal bio-char as a foaming agent for electric arc furnace steelmaking: performance and mechanism, Appl. Energy 353, 122084 (2024) [CrossRef] [Google Scholar]
- L. Kieush, J. Schenk, A. Koveria, A. Hrubiak, Biocoke thermochemical properties for foamy slag formations in electric arc furnace steelmaking, Metals 14, 13 (2023) [CrossRef] [Google Scholar]
- J.-X. Fu, C. Zhang, W.-S. Hwang, Y.-T. Liau, Y.-T. Lin, Exploration of biomass char for CO2 reduction in RHF process for steel production, Int. J. Greenhouse Gas Control 8, 143–149 (2012) [CrossRef] [Google Scholar]
- H. Han, D. Duan, P. Yuan, D. Li, Biomass reducing agent utilisation in rotary hearth furnace process for DRI production, Ironmak. Steelmak. 42, 579–584 (2015) [CrossRef] [Google Scholar]
- P. Yuan, B. Shen, D. Duan, G. Adwek, X. Mei, F. Lu, Study on the formation of direct reduced iron by using biomass as reductants of carbon containing pellets in RHF process, Energy 141, 472–482 (2017) [CrossRef] [Google Scholar]
- A. Babich, D. Senk, Coal use in iron and steel metallurgy, in: The Coal Handbook: Towards Cleaner Production (Elsevier, 2013), pp. 267–311 [CrossRef] [Google Scholar]
- A. Babich, D. Senk, H.W. Gudenau, Effect of coke reactivity and nut coke on blast furnace operation, Ironmak. Steelmak. 36, 222–229 (2009) [CrossRef] [Google Scholar]
- L. Florentino-Madiedo, E. Díaz-Faes, C. Barriocanal, Reactivity of biomass containing briquettes for metallurgical coke production, Fuel Process. Technol. 193, 212–220 (2019) [CrossRef] [Google Scholar]
- E. Mousa, C. Wang, J. Riesbeck, M. Larsson, Biomass applications in iron and steel industry: an overview of challenges and opportunities, Renew. Sustain. Energy Rev. 65, 1247–1266 (2016) [CrossRef] [Google Scholar]
- K.W. Ng, J.A. MacPhee, L. Giroux, T. Todoschuk, Reactivity of bio-coke with CO2, Fuel Process. Technol. 92, 801–804 (2011) [CrossRef] [Google Scholar]
- M. Castro-Díaz, C.N. Uguna, L. Florentino, E. Díaz-Faes, L.A. Stevens, C. Barriocanal, C.E. Snape, Evaluation of hydrochars from lignin hydrous pyrolysis to produce biocokes after carbonization, J. Anal. Appl. Pyrol. 124, 742–751 (2017) [CrossRef] [Google Scholar]
- X. Xing, Pore structure and integrity of a bio-coke under simulated blast furnace conditions, Energy Fuels 33, 2133–2141 (2019) [CrossRef] [Google Scholar]
- A. Koskela, Utilisation of lignin-based biocarbon in pyrometallurgical applications, PhD thesis, University of Oulu Graduate School; University of Oulu (2023). http://jultika.oulu.fi/files/isbn9789526236681.pdf (accessed December 11, 2023). [Google Scholar]
- BIOmass for COkemaking DEcarbonization, (2023). https://ec.europa.eu/info/funding-tenders/opportunities/portal/screen/how-to-participate/org-details/890031358/project/101112264/program/43252449/details (accessed November 14, 2023). [Google Scholar]
- R. Attrotto, BioCoDe. Biomass for cokemaking decarbonization, in: Barcelona, Spain (2023) [Google Scholar]
- S.P.E. Forsmo, S.-E. Forsmo, P.-O. Samskog, B.M.T. Björkman, Mechanisms in oxidation and sintering of magnetite iron ore green pellets, Powder Technol. 183, 247–259 (2008) [CrossRef] [Google Scholar]
- X. Fan, Z. Ji, M. Gan, X. Chen, T. Jiang, Integrated assessment on the characteristics of straw-based fuels and their effects on iron ore sintering performance, Fuel Process. Technol. 150, 1–9 (2016) [CrossRef] [Google Scholar]
- L. Lu, M. Adam, M. Kilburn, S. Hapugoda, M. Somerville, S. Jahanshahi, J.G. Mathieson, Substitution of charcoal for coke breeze in iron ore sintering, ISIJ Int. 53, 1607–1616 (2013) [CrossRef] [Google Scholar]
- T. Kawaguchi, M. Hara, Utilization of biomass for iron ore sintering, ISIJ Int. 53, 1599–1606 (2013) [CrossRef] [Google Scholar]
- M. Gan, X. Fan, Z. Ji, T. Jiang, X. Chen, Z. Yu, G. Li, L. Yin, Application of biomass fuel in iron ore sintering: influencing mechanism and emission reduction, Ironmak. Steelmak. 42, 27–33 (2015) [CrossRef] [Google Scholar]
- European Commission. Directorate General for Research and Innovation., Alternate carbon sources for sintering of iron ore (Acasos)., Publications Office, LU (2013). https://data.europa.eu/doi/10.2777/58105 (accessed November 9, 2023). [Google Scholar]
- S. Jahanshahi, J.G. Mathieson, M.A. Somerville, N. Haque, T.E. Norgate, A. Deev, Y. Pan, D. Xie, P. Ridgeway, P. Zulli, Development of low-emission integrated steelmaking process, J. Sustain. Metall. 1, 94–114 (2015) [CrossRef] [Google Scholar]
- T.C. Ooi, E. Aries, B.C.R. Ewan, D. Thompson, D.R. Anderson, R. Fisher, T. Fray, D. Tognarelli, The study of sunflower seed husks as a fuel in the iron ore sintering process, Minerals Eng. 21, 167–177 (2008) [CrossRef] [Google Scholar]
- G. Jha, S. Soren, K. Deo Mehta, Partial substitution of coke breeze with biomass and charcoal in metallurgical sintering, Fuel 278, 118350 (2020) [CrossRef] [Google Scholar]
- N.A. El-Hussiny, A.A. Khalifa, A.A. El-Midany, A.A. Ahmed, M.E.H. Shalabi, Effect of replacement coke breeze by charcoal on technical operation of iron ore sintering, J. Sci. Eng. Res. 6, 681–686 (2015) [Google Scholar]
- Z. Cheng, J. Yang, L. Zhou, Y. Liu, Z. Guo, Q. Wang, Experimental study of commercial charcoal as alternative fuel for coke breeze in iron ore sintering process, Energy Convers. Manag. 125, 254–263 (2016) [CrossRef] [Google Scholar]
- R.Z. Abd Rashid, H. Mohd. Salleh, M.H. Ani, N.A. Yunus, T. Akiyama, H. Purwanto, Reduction of low grade iron ore pellet using palm kernel shell, Renew. Energy 63, 617–623 (2014) [CrossRef] [Google Scholar]
- S. Luo, C. Ma, P. Sun, Reduction behavior and reaction kinetics of iron ore-biomass composite pellets, Chin. J. Eng. 37, 150–156 (2015) [Google Scholar]
- S. Ueda, K. Watanabe, K. Yanagiya, R. Inoue, T. Ariyama, Improvement of reactivity of carbon iron ore composite with biomass char for blast furnace, ISIJ Int. 49, 1505–1512 (2009) [CrossRef] [Google Scholar]
- A. Mousa, H. Ahmed, N. Viswanathan, M. Larsson, Recent trends in ironmaking blast furnace technology to mitigate CO2 emissions: tuyeres injection, in: Ironmaking and Steelmaking Processes: Greenhouse Emissions, Control and Reduction (Springer International Publishing: Switzerland, 2016), pp. 173–197 [CrossRef] [Google Scholar]
- M. Jeguirim, L. Limousy, eds., Char and carbon materials derived from biomass: production, characterization and applications ( Elsevier, Amsterdam, 2019) [Google Scholar]
- Y. Ueki, R. Yoshiie, I. Naruse, K. Ohno, T. Maeda, K. Nishioka, M. Shimizu, Reaction behavior during heating biomass materials and iron oxide composites, Fuel 104, 58–61 (2013) [CrossRef] [Google Scholar]
- J. Adilson De Castro, G.A.D. Medeiros, E.M.D. Oliveira, M.F. De Campos, H. Nogami, The mini blast furnace process: an efficient reactor for green pig iron production using charcoal and hydrogen-rich gas: a study of cases, Metals 10, 1501 (2020) [CrossRef] [Google Scholar]
- C.A. Scarpinella, T. Cyro, S.Y. Tagusagawa, M.B. Mourao, F.B. Lenz e Silva, Charcoal ironmaking: a contribution for CO2 mitigation, in: Metals and Materials Processing in a Clean Environment, Cancun, Mexico (2011) pp. 109–121 [Google Scholar]
- J. Orre, L.S. Ökvist, A. Bodén, B. Björkman, Understanding of blast furnace performance with biomass introduction, Minerals 11, 157 (2021) [CrossRef] [Google Scholar]
- J.A. De Castro, G.D.M. Araújo, I.D.O. Da Mota, Y. Sasaki, J. Yagi, Analysis of the combined injection of pulverized coal and charcoal into large blast furnaces, J. Mater. Res. Technol. 2, 308–314 (2013) [CrossRef] [Google Scholar]
- G. Wang, R. Li, J. Dan, X. Yuan, J. Shao, J. Liu, K. Xu, T. Li, X. Ning, C. Wang, Preparation of biomass hydrochar and application analysis of blast furnace injection, Energies 16, 1216 (2023) [CrossRef] [Google Scholar]
- L. Sundqvist Ökvist, M. Lundgren, Experiences of bio-coal applications in the blast furnace process—opportunities and limitations, Minerals 11, 863 (2021) [CrossRef] [Google Scholar]
- H. Suopajärvi, E. Pongrácz, T. Fabritius, The potential of using biomass-based reducing agents in the blast furnace: a review of thermochemical conversion technologies and assessments related to sustainability, Renew. Sustain. Energy Rev. 25, 511–528 (2013) [CrossRef] [Google Scholar]
- M. Rehfeldt, E. Worrell, W. Eichhammer, T. Fleiter, A review of the emission reduction potential of fuel switch towards biomass and electricity in European basic materials industry until 2030, Renew. Sustain. Energy Rev. 120, 109672 (2020) [CrossRef] [Google Scholar]
- L. Kieush, J. Schenk, A. Koveria, A. Hrubiak, H. Hopfinger, H. Zheng, Evaluation of slag foaming behavior using renewable carbon sources in electric arc furnace-based steel production, Energies 16, 4673 (2023) [CrossRef] [Google Scholar]
- E. Hoikkaniemi, P. Sulasalmi, V.-V. Visuri, T. Fabritius, Biochar as a slag foaming agent in EAF − a novel experimental setup, in: Oulu, Finland, 2023. [Google Scholar]
- A. Kalde, T. Demus, T. Echterhof, H. Pfeifer, Determining the reactivity of biochar-agglomerates to replace fossil coal in electric arc furnace steelmaking, in: Proceedings of the EUBCE 2015 Online Conference Proceedings (2015) pp. 497–507 [Google Scholar]
- I. Shukla, Potential of renewable agricultural wastes in the smart and sustainable steelmaking process, J. Cleaner Prod. 370, 133422 (2022) [CrossRef] [Google Scholar]
- N.F.M. Yunos, M. Zaharia, M.A. Idris, D. Nath, R. Khanna, V. Sahajwalla, Recycling agricultural waste from palm shells during electric arc furnace steelmaking, Energy Fuels 26, 278–286 (2012) [CrossRef] [Google Scholar]
- B. Fidalgo, C. Berrueco, M. Millan, Chars from agricultural wastes as greener fuels for electric arc furnaces, J. Anal. Appl. Pyrol. 113, 274–280 (2015) [CrossRef] [Google Scholar]
- C. DiGiovanni, D. Li, K.W. Ng, X. Huang, Ranking of injection biochar for slag foaming applications in steelmaking, Metals 13, 1003 (2023) [CrossRef] [Google Scholar]
- X.-A. Huang, K.W. Ng, L. Giroux, M. Duchesne, Carbonaceous material properties and their interactions with slag during electric arc furnace steelmaking, Metall. Mater. Trans. B 50, 1387–1398 (2019) [CrossRef] [Google Scholar]
- A. Funke, T. Demus, T. Willms, L. Schenke, T. Echterhof, A. Niebel, H. Pfeifer, N. Dahmen, Application of fast pyrolysis char in an electric arc furnace, Fuel Process. Technol. 174, 61–68 (2018) [CrossRef] [Google Scholar]
- Sustainable EAF steel production (GREENEAF) (2013). https://op.europa.eu/en/publication-detail/-/publication/e7dc500c-82de-4c2d-8558-5e24a2d335fb (accessed November 13, 2023) [Google Scholar]
- F. Cirilli, G. Baracchini, L. Bianco, EAF long term industrial trials of utilization of char from biomass as fossil coal substitute, La Metallurgia Italiana 109, 13–17 (2017) [Google Scholar]
- T. Meier, T. Hay, T. Echterhof, H. Pfeifer, T. Rekersdrees, L. Schlinge, S. Elsabagh, H. Schliephake, Process modeling and simulation of biochar usage in an electric arc furnace as a substitute for fossil coal, Steel Research Int. 88, 1600458 (2017) [CrossRef] [Google Scholar]
- C. Wang, Y.-C. Lu, L. Brabie, G. Wang, A pilot trial investigation of using hydrochar derived from biomass residues for EAF process, in Advances in Pyrometallurgy, edited by C. Fleuriault, J.D. Steenkamp, D. Gregurek, J.F. White, Q.G. Reynolds, P.J. Mackey, S.A.C. Hockaday (Springer Nature Switzerland, Cham, 2023 ) pp. 153–163 [CrossRef] [Google Scholar]
- E. Sandberg, Pilot tests with use of secondary material streams as replacement of fossil carbon and burnt lime in EAF steelmaking, in: Barcelona, Spain (2023) [Google Scholar]
- H. Mandova, W.F. Gale, A. Williams, A.L. Heyes, P. Hodgson, K.H. Miah, Global assessment of biomass suitability for ironmaking − opportunities for co-location of sustainable biomass, iron and steel production and supportive policies, Sustain. Energy Technolog. Assess. 27, 23–39 (2018) [CrossRef] [Google Scholar]
- B. Brooks, S.K. Rish, H. Lomas, A. Jayasekara, A. Tahmasebi, Advances in low carbon cokemaking − influence of alternative raw materials and coal properties on coke quality, J. Anal. Appl. Pyrol. 173, 106083 (2023) [CrossRef] [Google Scholar]
- J. Cai, G. Yu, H. Liao, K. Qian, P. Zhao, Y. He, Disposal of waste plastics with traditional coking process, J. Iron Steel Res. Int. 13, 5–9 (2006) [CrossRef] [Google Scholar]
- S. Devasahayam, G. Bhaskar Raju, C. Mustansar Hussain, Utilization and recycling of end of life plastics for sustainable and clean industrial processes including the iron and steel industry, Mater. Sci. Energy Technolog. 2, 634–646 (2019) [Google Scholar]
- S. Nomura, Behavior of chlorine during co-carbonization of coal and chloride compounds in cokemaking process, Int. J. Coal Geol. 130, 27–32 (2014) [CrossRef] [Google Scholar]
- S. Nomura, The effect of plastic addition on coal caking properties during carbonization, Fuel 82, 1775–1782 (2003) [CrossRef] [Google Scholar]
- S. Nomura, Use of waste plastics in coke oven: a review, J. Sustain. Metall. 1, 85–93 (2015) [CrossRef] [Google Scholar]
- S. Nomura, K. Kato, The effect of plastic size on coke quality and coking pressure in the co-carbonization of coal/plastic in coke oven, Fuel 85, 47–56 (2006) [CrossRef] [Google Scholar]
- S. Nomura, K. Kato, Basic study on separate charge of coal and waste plastics in coke oven chamber, Fuel 84, 429–434 (2005) [CrossRef] [Google Scholar]
- M. Knepper, A. Babich, D. Senk, T. Buergler, C. Feilmayr, N. Kieberger, Waste plastics injection: reaction kinetics and effect on the blast furnace process, in: Rio de Janeiro, RJ, Brazil (2012) pp. 798–810 [Google Scholar]
- A. Babich, D. Senk, M. Knepper, S. Benkert, Conversion of injected waste plastics in blast furnace, Ironmak. Steelmak. 43, 11–21 (2016) [CrossRef] [Google Scholar]
- S. Devasahayam, Review: opportunities for simultaneous energy/materials conversion of carbon dioxide and plastics in metallurgical processes, Sustain. Mater. Technolog. 22, e00119 (2019) [Google Scholar]
- I. Bellemans, K. Verbeken, Towards circularity in steel industry: a joint journey between industry and universities along multiple TRL levels, in: Barcelona, Spain, 2023. [Google Scholar]
- S. Maroufi, M. Mayyas, I. Mansuri, P. O’Kane, C. Skidmore, Z. Jin, A. Fontana, V. Sahajwalla, Study of reaction between slag and carbonaceous materials, Metall. Mater. Trans. B 48, 2316–2323 (2017) [CrossRef] [Google Scholar]
- M. Zaharia, V. Sahajwalla, B.-C. Kim, R. Khanna, N. Saha-Chaudhury, P. O’Kane, J. Dicker, C. Skidmore, D. Knights, Recycling of rubber tires in electric arc furnace steelmaking: simultaneous combustion of metallurgical coke and rubber Tyres blends, Energy Fuels 23, 2467–2474 (2009) [CrossRef] [Google Scholar]
- V. Sahajwalla, Recycling Waste Plastics in EAF Steelmaking: Carbon/Slag Interactions of HDPE-Coke Blends (Steel Research International, 2009) [Google Scholar]
- S. Kongkarat, R. Khanna, P. Koshy, P. O’kane, V. Sahajwalla, Recycling waste polymers in EAF steelmaking: influence of polymer composition on carbon/slag interactions, ISIJ Int. 52, 385–393 (2012) [CrossRef] [Google Scholar]
- EAF working with polymers derived from plastic residue in substitution of fossil fuel (2020). https://ec.europa.eu/info/funding-tenders/opportunities/portal/screen/how-to-participate/org-details/962112446/project/899415/program/31061225/details (accessed November 15, 2023). [Google Scholar]
- I. Kofler, M. Derntl, Pilot demonstration of carbon capture and utilization technology to close the carbon cycle in the steel industry. Presented at A Circular Economy Driven by European Steel, in: Barcelona, Spain (2023) [Google Scholar]
- P. Brandl, M. Bui, J.P. Hallett, N. Mac Dowell, A century of re-exploring CO2 capture solvents, Int. J. Greenhouse Gas Control 120, 103771 (2022) [CrossRef] [Google Scholar]
- S. Manocha, CO2 (CCUS) focus on capture & usage, in: Barcelona, Spain (2023) [Google Scholar]
- V. Colla, I. Matino, T.A. Branca, B. Fornai, L. Romaniello, F. Rosito, Efficient use of water resources in the steel industry, Water 9, 874 (2017) [CrossRef] [Google Scholar]
- I. Matino, V. Colla, A. Maddaloni, S. Cateni, V. Iannino, A. Petrucciani, A. Zaccara, T.A. Branca, R. Matino, M. Chini, L. Bianco, S. Porisiensi, L. De Cecco, G. Tomat, F. Nodusso, G. Lepore, Decreasing the use of high-quality make-up water in the steel sector by coupling enhanced sensors circuit with decision and support tool, Water 15, 3208 (2023) [CrossRef] [Google Scholar]
- T.A. Branca, V. Colla, M.I. Pistelli, L.E. Faraci, F. Cirilli, A. Schröder, Effects of industrial symbiosis and energy efficiency in terms of new skills requirement in the steel sector, in: Barcelona, Spain (2023) [Google Scholar]
- Worldsteel Association. Fact sheet “Working in the steel industry,” (2021). https://worldsteel.org/wp-content/uploads/Fact-sheet-Working-in-the-steel-industry.pdf (accessed November 14, 2023). [Google Scholar]
- H. Oterdoom, European steel: playing a key role in technological innovation, self-reliance, ethics, and circularity of more than steel alone, in: Barcelona, Spain (2023) [Google Scholar]
- A. Schröder, Skills for industrial symbiosis and energy efficiency, in: Barcelona, Spain (2023) [Google Scholar]
- European Steel Technology Platform (ESTEP): Clean Steel Partnership Roadmap (2021). https://www.estep.eu/assets/Uploads/CSP-SRIA-Oct2021-clean.pdf (accessed November 9, 2023). [Google Scholar]
- C. Pietrosanti, V. Colla, Digital tools pave the way to Circular Economy in the European steel sector through new business models, in: Barcelona, Spain (2023) [Google Scholar]
- T. Adisorn, L. Tholen, T. Götz, Towards a digital product passport fit for contributing to a circular economy, Energies 14, 2289 (2021) [CrossRef] [Google Scholar]
- Worldsteel Association. Fact sheet “Scrap use in the steel industry,” (2021). https://worldsteel.org/wp-content/uploads/Fact-sheet-on-scrap_2021.pdf (accessed November 14, 2023) [Google Scholar]
Cite this article as: Lina Kieush, Johannes Rieger, Rosella Attrotto, Angelo Sorino, Wim van der Stricht, Harmen Oterdoom, Eetu Pekka Heikkinen, Gianluca Dall’Osto, Carlo Mapelli, Davide Mombelli, Loredana Di Sante, Filippo Cirilli, Valentina Colla, Teresa Annunziata Branca, Ismael Matino, Alice Petrucciani, Antonella Zaccara, Carlo Brondi, Elsayed Mousa, Erland Nylund, Erik Sandberg, Marta Guzzon, Enrico Malfa, Antonius Schröder, Inge Bellemans, Roadmap for recycling practices and resource utilization in the iron and steelmaking industry: a case studies, Matériaux & Techniques 112, 503 (2024)
All Tables
All Figures
 |
Fig. 1 Schematic of slag generation in a BF, BOF, EAF, and LF, adapted from Ref. [26], Copyright (2015), with permission from Elsevier. |
| In the text | |
 |
Fig. 2 Overview of residue possibilities from steel plants under the ReMFra project [48]. |
| In the text | |
 |
Fig. 3 Schematic representation of scrap recycling, adapted from Ref. [52], Copyright (2014), with permission from Elsevier. |
| In the text | |
 |
Fig. 4 Steelanol (a); and Torero (b) demonstration plants. |
| In the text | |
 |
Fig. 5 BIOCode project activities and objectives, reproduced from Ref. [106]. |
| In the text | |
 |
Fig. 6 Pathways to produce reducing agents for the metallurgical industry from biomass by thermochemical conversion, reproduced from Ref. [130], Copyright (2013), with permission from Elsevier. |
| In the text | |
 |
Fig. 7 Austrian-funded ZEUS project to demonstrate a cross-sectoral climate-neutral carbon cycle for accelerating the technology transfer into practice, reproduced from Ref. [164]. |
| In the text | |
 |
Fig. 8 A simplified flowsheet of the water circuit considered in the rolling mill use case, reproduced from Ref. [168]. |
| In the text | |
 |
Fig. 9 Generic job profiles are generated by the sectors involved in the SPIRE-SAIS project (HR is Human Resources; OHS is Occupational Health and Safety), reproduced from Ref. [172]. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.







