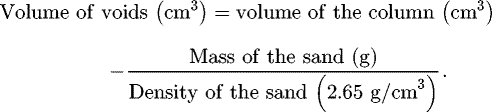| Numéro |
Matériaux & Techniques
Volume 108, Numéro 3, 2020
Impact of microorganisms on cementitious materials
|
|
|---|---|---|
| Numéro d'article | 302 | |
| Nombre de pages | 13 | |
| Section | Biomatériaux / Biomaterials | |
| DOI | https://doi.org/10.1051/mattech/2020027 | |
| Publié en ligne | 18 novembre 2020 | |
Regular Article
Biomineralization of calcium carbonate by marine bacterial strains isolated from calcareous deposits
Biominéralisation du carbonate de calcium par des souches bactériennes marines isolées de dépôts calco-magnésiens
1
LIENSs UMR 7266 CNRS La Rochelle Université,
avenue M. Crépeau,
17000
La Rochelle, France
2
LaSIE UMR 7356 CNRS La Rochelle Université,
avenue M. Crépeau,
17000
La Rochelle, France
* e-mail: julia.vincent1@univ-lr.fr
Received:
20
December
2019
Accepted:
9
September
2020
Biomineralization induced by microbial enzymes, which catalyse CaCO3 precipitation, is a promising field of research for various applications in building eco-materials. Especially, this could provide an eco-friendly process for protection of coastal areas against erosion. In the present investigation, fourteen bacterial strains were isolated and characterized from both natural seawater and calcareous deposits formed on a cathodically protected steel mesh in marine environment. All of them induced calcium carbonate precipitation in various media by producing urease and/or carbonic anhydrase enzymes. The calcium carbonate minerals produced by bacteria were identified by microscopy and µ-Raman spectroscopy. In parallel, an experimental set-up, based on a column reactor, was developed to study biomineralization and microbial capacity of Sporosarcina pasteurii to form sandy agglomerate. These well-known calcifying bacteria degraded the urea present in liquid medium circulating through the column to produce calcium carbonate, which acted as cement between sand particles. The bio-bricks obtained after 3 weeks had a compressive strength of 4.2 MPa. 20% of the inter-granular voids were filled by calcite and corresponded to 13% of the total mass. We successfully showed that bio-column system can be used to evaluate the bacterial ability to agglomerate a sandy matrix with CaCO3.
Résumé
La biominéralisation induite par des enzymes microbiennes qui catalysent la précipitation du CaCO3 est un domaine de recherche prometteur pour diverses applications dans les éco-matériaux de construction. Cela pourrait en particulier permettre d’établir un procédé écologique de protection des zones côtières contre l’érosion. Dans le cadre de la présente étude, quatorze souches bactériennes ont été isolées à partir d’eau de mer naturelle et de dépôts calcaires formés sur une grille en acier placée sous protection cathodique en milieu marin. Toutes ces bactéries ont induit la précipitation de carbonate de calcium dans divers milieux en produisant des enzymes uréase et/ou anhydrase carbonique. Les minéraux de carbonate de calcium produits par les bactéries ont été identifiés par microscopie et par spectroscopie µ-Raman. En parallèle, un dispositif expérimental, basé sur un réacteur en colonne, a été développé pour étudier la biominéralisation et la capacité microbienne de Sporosarcina pasteurii à former un agglomérat sableux. Ces bactéries calcifiantes bien connues ont dégradé l’urée présente dans le milieu liquide circulant dans la colonne pour produire du carbonate de calcium qui a agi comme un ciment entre les particules de sable. Les bio-briques obtenues au bout de trois semaines avaient une résistance à la compression de 4,2 MPa. Vingt pour cent des vides intergranulaires ont été remplis de calcite et correspondent à 13 % de la masse totale. Nous avons réussi à montrer que le système de bio-colonne peut être utilisé pour évaluer la capacité des bactéries à agglomérer une matrice sableuse avec du CaCO3.
Key words: biomineralization / calcareous deposits / marine bacteria / calcium carbonate precipitation / bio-bricks
Mots clés : biominéralisation / dépôts calco-magnésiens / bactérie marine / précipitation du carbonate de calcium / bio-briques
© SCF, 2020
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
1 Introduction
Coastal erosion poses a severe threat as it causes degradation of dunes, beaches, cliffs and breakdown of dikes. Coastal erosion is recognized as the permanent loss of land along the shoreline resulting in the transformation of the coast. Over the last 50 years, nearly 20% of the French natural coastline has receded and about 30 km2 of land have disappeared [1]. In order to protect coastal areas and structures, it is necessary to find an effective means of fighting erosion with a low impact on the environment. The possibility way to promote the formation of an eco-material by electrochemistry has been envisioned as a means to stabilize the profile of beaches and to improve the anchoring of rocks used as breakwaters [2]. The process uses cathodic polarization of a metal structure that promotes the formation of a calcareous layer, composed of calcium carbonate (CaCO3) and magnesium hydroxide (Mg(OH)2), close to the metal. These calcareous deposits act as a cement between sediments, shells, and pebbles, and give rise to a solid agglomerate that could reinforce dikes and delay the erosion of beaches. As this process evolves in a natural marine environment, the presence of numerous microorganisms could also influence the formation of calcareous deposits and it is necessary to take this into account. Indeed, some microorganisms are able to promote a mineral precipitation, called biomineralization. A local microenvironment is generated by microorganisms, which provides optimal conditions for the mineral precipitation [3]. In marine environment, this phenomenon is responsible, in the long term, for the formation of rocks such as stromatolites [4] or beachrocks [5,6]. Calcium carbonate is one of the most abundant minerals on earth, comprising 4% in weight of the earth’s crusts [7]. Several microbial metabolic processes are able to induce the precipitation of calcium carbonate minerals (biocalcification) both in marine and terrestrial environments: urea hydrolysis, ammonification of amino acids, denitrification, dissimilatory sulphate reduction and photosynthesis [7–9]. The most widely studied metabolic pathway is urea hydrolysis driven by bacterial urease, which leads to an alkalization of the medium inducing precipitation of CaCO3 [8,10–12]. This metabolism could be applied in particular to the repair of concrete building materials [13], the stabilization of soils [14], the depollution of materials contaminated with heavy metals [15] and the consolidation of sandy substrate [16,17]. Carbonic anhydrase enzymes are also involved in the formation of CaCO3 precipitates under natural conditions and can act synergistically with urease [18,19]. Carbonic anhydrase promotes the interconversion of CO2 into HCO3− and H+, which under certain conditions can capture atmospheric CO2 [20,21]. In the context of coastal protection, our purpose is to design eco-materials that mimic naturally-formed rock structures at the seaside and to accelerate their growth kinetics by coupling electrochemical processes with microbial mechanisms of biomineralization. To do this, it is necessary in a first step to identify marine bacteria able to induce biomineralization and more specifically biocalcification. In this work, fourteen marine bacteria have been isolated from both natural seawater and calcareous deposits formed on a cathodically protected steel mesh in marine environment. The mineralization products and metabolic pathway involved in biocalcification have been characterized. Simultaneously, an experimental set-up of bio-solid column has been developed and validated by using a well-known calcifying bacterium, Sporosarcina pasteurii DSM33, able to agglomerate a sandy substrate. The calcareous-sand agglomerate obtained has been mechanically tested.
2 Materials and methods
In order to explicit the various types of experiments and the links between them, the experimental procedure is summarized in Figure 1.
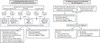 |
Fig. 1 Experimental procedure for obtaining bacteria able to carry out biomineralization in the marine environment (A) and setting up a reactor for manufacturing bio-bricks (B). X: no colonies with mineral precipitation on their surface. |
2.1 Culture media and solutions
Two different media were used to isolate and cultivate marine bacteria:
-
the first one, Marine Broth (MB) (Condalab), was composed of ammonium nitrate 0.0016 g/L, bacteriological peptone 5 g/L, boric acid 0.022 g/L, anhydrous calcium chloride 1.8 g/L, disodium phosphate 0.008 g/L, anhydrous magnesium chloride 8.8 g/L, potassium bromide 0.08 g/L, potassium chloride 0.55 g/L, sodium bicarbonate 0.16 g/L, sodium chloride 19.4 g/L, sodium fluoride 0.0024 g/L, sodium silicate 0.004 g/L, sodium sulphate 3.24 g/L, strontium chloride 0.034 g/L, yeast extract 1 g/L, ferric citrate 0.1 g/L. From this one, two media were produced: MB enriched with calcium chloride dihydrate at the concentration of 3.7 g/L (named MB + CaCl2) and MB enriched with calcium chloride dihydrate at the concentration of 3.7 g/L and urea at the concentration of 20 g/L (named MB + CaCl2 + urea);
-
the second medium, M1, was adapted from the study of Silva-Castro et al. [22]. Its composition was yeast extract 10 g/L, peptone 5 g/L, glucose 1 g/L and sea salts (Sigma) 30 g/L. From this one, two media were produced: M1 enriched with calcium chloride dihydrate at the concentration of 3.7 g/L (named M1 + CaCl2) and M1 enriched with calcium chloride dihydrate at the concentration of 3.7 g/L and urea at the concentration of 20 g/L (named M1 + CaCl2 + urea).
The media used to cultivate non-marine bacteria for the control experiments and the bio-solid column were:
-
medium 220 (M220) composed of peptone from casein 20 g/L, sodium chloride 5 g/L, with urea at the concentration of 20 g/L;
-
tryptic Soy Broth (TSB) composed of tryptone 17 g/l, papaic digest of soybean meal 3 g/L, glucose 2.5 g/L, dipotassium phosphate 2.5 g/L, sodium chloride 5 g/L, pH of the ready use medium (25 °C) 7.3 ± 0.2.
All these media were solidified by adding agar 12 g/L.
Artificial seawater (ASW) based on the standard ASTM D1141 (Standard specification for substitute ocean water, corrosion testing procedures, 1992) was prepared for isolation of marine bacteria. It was composed of sodium chloride 24.54 g/L, calcium chloride dihydrate 1.54 g/L, magnesium chloride hexahydrate 11.1 g/L, sodium sulphate 4.09 g/L, potassium chloride 0.69 g/L and sodium carbonate 0.23 g/L.
Cementation medium, used for the bio-solid column, was composed of Nutrient Broth (NB) 0.5 g/L, enriched with calcium chloride dihydrate at the concentration of 3.7 g/L and urea at the concentration of 20 g/L, at the pH of 7.5.
2.2 Sampling, bacterial strains and culture conditions
To isolate marine bacteria inducing biomineralization, samples were collected from two different sites. Natural seawater and calcareous deposits developed by cathodic polarization on steel grid during 6 months were sampled in La Rochelle harbour (Atlantic coast, France). Natural seawater and mud were also sampled on the Atlantic coast in Angoulins (village close to La Rochelle, France).
Approximately 4 g of calcareous deposits were placed in 50 mL of MB + CaCl2, MB + CaCl2 + urea, M1 + CaCl2, M1 + CaCl2 + urea and incubated at 25 °C, with shaking of 130 rpm, for 1 week. For each culture condition, bacteria were isolated by the serial dilution method consisting of plating the samples on solid media MB + CaCl2, MB + CaCl2 + urea, M1 + CaCl2, M1 + CaCl2 + urea, and incubated for 3 days at 25 °C.
Bacterial strains contained in natural seawater of both sites and in mud from Angoulins were isolated by the serial dilution method in ASW solution by plating the samples on solid medium M1 + CaCl2 for 3 days at 25 °C.
Bacterial strains with mineral precipitation on the surface of colonies were isolated and characterized. Strains were conserved as frozen stocks with 25% glycerol at −80 °C. For all tests, the bacteria were grown at 30 °C in their respective isolation medium.
Sporosarcina pasteurii DSM33, used for the biocalcification assays in bio-solid column, was cultivated in M220 medium + 2% urea at 30 °C. Escherichia coli ATCC 25922 and Proteus mirabilis ATCC 25933, used as controls for urease activity assay, were cultivated in TSB medium at 37 °C, corresponding to optimal growth temperature.
2.3 Morphological characterization of isolated bacterial strains
Morphological characterization of colonies and cells was performed by standard methods.
Bacterial colonies were observed with binocular magnifying glass Leica M165C (x1.25 to x12).
Gram stain for light microscopy was done on heat-fixed smears of bacteria according to the following procedure. The bacteria were flooded with a dye (crystal violet) for 60 seconds, then flooded with a mordant (lugol solution) for an additional 60 seconds to fix the staining, and gently washed under running tap water. Bacteria were decolorized with ethanol 95°, washed with water and counterstained with fuchsin for 60 seconds. Cells were then washed with water, blotted dry, and observed by light microscopy (Leica microscope LEITZ Biomed, x1000).
For the motility experiment, bacterial suspensions were prepared with the MB medium inoculated with bacteria, incubated at 30 °C with shaking of 130 rpm for 24 hours. A droplet was placed between a lamella and a glass slide. The motility was observed with Leica microscope LEITZ Biomed (x1000).
2.4 Respiratory type of isolated bacterial strains
Each isolated strain was inoculated on the surface of respective solid medium in aerobic and anaerobic conditions and was incubated at 25 °C for 3 days. The anaerobic conditions were realised by using GasPak™ EZ Anaerobe container system (Becton, Dickinson and company) in airtight container.
2.5 Determination of medium pH
pH of samples was measured with a WTW pH-meter 3310 with electrode SENTIX Mic-D (WTW).
2.6 Urease activity assay
Indole urea solution (Biomérieux) was used to determine bacterial urease activity. It is composed of L-tryptophan 3 g/L, potassium dihydrogen phosphate 1 g/L, dipotassium hydrogen phosphate 1 g/L, sodium chloride 5 g/L, urea 20 g/L, phenol red 0.025 g/L, alcohol 95° 0.01 ml/L and final pH (at 25 °C) 6.8 ± 0.2. Several colonies of each isolated bacterium were inoculated in 1 mL of indole urea medium. Samples were placed at 30 °C for 24 hours. The ability of the bacterial isolates that produce urease leads to NH4+ release and then increases the pH into alkaline domain. As a result, the colour of the medium turns pink. Escherichia coli ATCC 25922 was used as negative control of urease activity and Sporosarcina pasteurii DSM 33 and Proteus mirabilis ATCC 25933 were used as positive controls of urease activity.
2.7 Carbonic anhydrase activity assay
The assay of carbonic anhydrase (CA) activity was carried out with para nitrophenol acetate (p-NPA), which is a colour indicator. A solution of 20 mM phosphate buffer and 1 mol/L p-NPA was used for this assay. Bacterial colonies, grown on solid medium for 24 hours, were put in 3 ml of reaction solution and then incubated at ambient temperature for 1 hour. The esterase activity of CA was measured by colour change of the solution induced by hydrolysis of p-NPA used as CA substrate. Negative control was done with 3 mL of reaction solution and sterile culture medium without bacteria. Escherichia coli ATCC 25922 was used as positive control of CA activity.
2.8 Crystals morphology
Isolated bacterial strains grew on respective solid isolation media enriched in calcium (MB + CaCl2 or M1 + CaCl2) for 3 days at 25 °C. Morphology of mineralization products at the surface of the colonies was observed with binocular magnifying glass Leica M165C (x1.25 to x12).
2.9 Crystals identification by µ-Raman spectroscopy
µ-Raman spectroscopy analysis was performed at room temperature on a Jobin Yvon High Resolution Raman Spectrometer (LabRAM HR Evo) equipped with a microscope (Olympus BX 41), a Peltier-based cooled charge coupled detector (CCD) and a Near Infra-Red diode laser (785 nm). The acquisition time was variable and depended on fluorescence background of the spectrum due to the organic matter. It was generally equal to 60 seconds but could be increased up to 5 minutes to optimize the signal to noise ratio. At least 5 or 6 zones (diameter of approximately 2 µm) of a given deposit were analysed through a Long Working Distance 50 × objective, with a resolution estimated at 0.1 cm.
2.10 Experimental set-up of bio-solid column
An experimental set-up based on a column reactor was built to study biomineralization and microbial capacity to consolidate sand. The scheme is shown in Figure 2. This experimental set-up has been adapted from experimentations of Henze and Randall [23]. The column had an inside diameter of 44.5 mm and a length of 150 mm. The bottom and top lids were identical and removable, both sealed with an O-ring. Additionally, a scrub sponge and a filter were cut into a cylindrical shape with a diameter of 44 mm and placed on both sides of the column. A 0/4 mm pure SiO2 sand (from Kleber Moreau Roussillon aggregates quarry), which is similar to that of masonry sand, was used for these experiments. It was sterilised at 115 °C for 20 min.
Bacterial culture was prepared with Sporosarcina pasteurii DSM33 in 100 ml of M220 medium + 2% urea and incubated for 24 hours. For the inoculation of the sand with bacteria, 350 g of sand was mixed with this bacterial culture and shaked slowly (60 rpm) for 4 hours at 30 °C, to favour bacterial adhesion to the sand. The sand-bacteria mixture was then added to the column. The remaining liquid was drained through the outlet. The column was then closed and bacteria were kept for 3 hours. The system was connected through a peristaltic pump to a bottle containing sterile cementation medium. 50 mL of cementation medium was injected every 3 hours at a rate of 10ml/min during 3 weeks from the bottom of the column. The liquid that flowed out on the top of the column was collected in order to check the pH.
2.11 Mechanical and physical analyses of the cemented sandy column
Axial compressive test and accessible water porosity were performed on a cylindrical calcareous agglomerate of 43.2 mm in diameter and 50.2 mm in height that have been sawn with a circular saw from the top of the column. The difference between the agglomerate diameter and the internal diameter of the column was due to the column drying (one week at 37 °C). The axial compressive test consisted of increasing the load progressively at the rate 50 N/s until the fracture (one replicate). The compressive strength in megapascals (MPa) is considered as the ratio between the maximum load at fracture (Fc) and the area where the load is applied (Ac). The accessible water porosity was measured according to the AFPC-AFREM procedure and assessed from water saturation under vacuum (AFPC-AFREM 1997). Specimens were water-saturated during 24 h under vacuum (around 2.5 kPa) and then oven-dried at 105 °C. Volumes were determined by hydrostatic weighing. The water porosity is then defined as the intergranular voids volume on total volume ratio and was averaged with 8 replicates. The initial intergranular porosity, said to be accessible to water, corresponds to the initial void volume and is expressed as a function of the total volume of the sample:

where
Thermogravimetric analysis (TGA) was performed on a Setaram Setsys Evolution 16/18 apparatus. 241.2 mg of samples were heated from 20 °C to 1000 °C at a 10 °C/min heating rate, under neutral argon atmosphere. To get more accurate results, data were analysed through the differential thermogravimetric curves. TGA results provide the CaCO3 content of the tested sample.
3 Results and discussion
3.1 Isolation and characterization of marine bacteria inducing biomineralization
Calcareous deposits, seawater and mud samples were taken in order to isolate bacterial strains able to induce biomineralization. After 1 week in two culture media (MB + CaCl2 and M1 + CaCl2), bacteria were deposited on the respective agar media (Fig. 1). After growth for 3 days, among all the bacteria that developed, only 14 colonies presented mineral precipitation on their surface. These bacteria were then isolated on the respective agar media (see bacterial colonies in Fig. 3). Isolated strains were denoted CDi (i = 1 to 10) for bacteria taken from the calcareous deposit (CD), Md1 for bacteria coming from the mud in Angoulins coast, and SWi (i = 1 to 3) for bacteria from seawater sampled in La Rochelle harbour or Angoulins coast (Fig. 1).
The 14 marine strains have been characterized according to their morphotype by Gram coloration, their respiratory type, their motility and the pH of their culture medium (Tab. 1). We mostly observed motile bacilli types with a majority of Gram-negative bacteria (71%). This result is consistent with the predominance of Proteobacteria among the bacterial communities in seawater [24,25]. In our work, twelve of the isolated bacteria were facultative anaerobe (FA) and two were obligate aerobe (OA). The pH of the liquid culture medium MB + CaCl2 or M1 + CaCl2 (according to the strains, see Tab. 1), initially at 7.5 ± 0.1, increased to 7.9 ± 0.1 for CD6, CD9, CD10, SW3 and up to 8.2 ± 0.2 for CD8, after 3 days of incubation at 25 °C. All isolated strains alkalinized their culture medium. Indeed, the bacteria responsible for the phenomenon of biocalcification are known to induce alkalization of their environment to favour the mineral precipitation [7,8].
By their metabolism, these isolated strains were responsible for cell environment alkalinisation, allowing them to precipitate mineral agglomerates on the surface of their colonies during their growth on solid medium.
 |
Fig. 3 Colonies of marine bacterial strains with mineral crust on the surface of the colonies. CD1 to CD8 were isolated on solid MB + CaCl2, other bacteria were isolated on solid M1 + CaCl2 for 3 days at 25 °C. Arrows indicate mineral agglomerate induced by bacteria. |
Characterization of isolated bacterial strains inducing mineral agglomerates on the surface of the colonies.
3.2 Identification of CaCO3-precipitating bacteria and characterization of the precipitated crystals in solid and liquid media
Optical microscopy and µ-Raman spectroscopy were used to characterize the minerals produced by the 14 marine bacterial strains studied and by the biocalcifying bacterium model, Sporosarcina pasteurii DSM 33, grown in several solid and liquid culture media: MB + CaCl2, MB + CaCl2 + urea, M1 + CaCl2, M1 + CaCl2 + urea. All bacterial strains produced a high number of crystals on solid agar after 3 days whereas no crystals were found in control plates inoculated without bacteria. Figure 4 shows crystals formed on the surface of colonies grown on solid culture media and observed through a binocular magnifying glass.
The most common crystal morphology was dumbbell shape. It was observed for CD1, CD3, CD8, CD9, CD10, Md1, SW1, SW2, SW3. For CD2, CD4, CD7 strains, we observed crystals forming contiguous spheres. Crystals of strains CD5 and CD6 were of irregular form. The crystal length of isolated marine strains was between 30 μm for CD7 and 100 μm for Md1 (Fig. 4). Crystal morphology of all isolated marine bacteria did not correspond with that of spherical crystals (30 μm) formed on the surface of Sporosarcina pasteurii DSM 33 for the same incubation time.
Some of the crystals present on the surface of bacterial colonies were collected and analysed by µ-Raman spectroscopy. Raman spectra of crystals from three strains, CD8, CD9 and Sporosarcina pasteurii DSM 33, are shown as examples in Figure 5.
All Raman spectra exhibited a vibration band around 1085 cm−1 corresponding to the carbonate symmetric vibration mode of CaCO3 (aragonite or calcite). In the case of Sporosarcina pasteurii, aragonite was identified via Raman vibration peaks at 155, 206 and a doublet at 707 cm−1 (Fig. 5b). For all other crystals obtained from marine bacterial strains, either calcite or an undefined CaCO3 was identified. Calcite is the most stable form of CaCO3 and exhibits vibration bands at 1085 and 155 cm−1 as for aragonite, but with two other typical vibration bands at 280 and 714 cm−1. Spectrum of Figure 5a obtained from CD9 bacterial strain corresponded to calcite although some slight peak shifts were observed probably due to the intense fluorescence background observed. This fluorescence background due to organic matter (biofilm, bacterial cells or remaining culture medium) was sometimes so intense that only the most intense vibration band of carbonate at 1081 cm−1 was visible (Fig. 5c). As it was not possible to identify the allotropic form of limescale, it was named as undefined type-CaCO3.
All the results for the fourteen biocalcifying bacteria isolated and grown in several solid and liquid culture media (MB + CaCl2, MB + CaCl2 + urea, M1 + CaCl2, M1 + CaCl2 + urea) are listed in Table 2.
Characterization of these precipitates showed that calcite was the main CaCO3 form produced by the strains under the different culture conditions. In the liquid and solid MB + CaCl2 with and without urea, all the isolated marine strains produced calcite. Moreover, in solid medium MB + CaCl2 with and without urea, the shapes of crystals for CD1, CD2, CD4, CD5 and CD7 were different depending on the presence or absence of urea. In the M1 + CaCl2 medium with and without urea, only CD9, CD10, Md1, SW1, SW2 and SW3 produced calcite in solid and liquid conditions. The other strains did not produce CaCO3 in solid condition except CD8 that showed an undefined type CaCO3 when urea was added. This can be explained by the absence in the medium of a compound necessary for biocalcification. Nevertheless, in liquid condition with and without urea, some bacteria produced CaCO3 but the spectrum obtained by Raman spectroscopy did not precisely allow to identify the CaCO3 compound obtained.
It is reported that the composition of the medium, culture methods and bacterial isolates can control the type of crystals produced [26,27]. This could explain why Sporosarcina pasteurii DSM33 induced the formation of aragonite in the medium M220 + 2% urea whereas this strain induced calcite formation in the medium MB + CaCl2 + urea. In different cultures of marine bacteria, the size of crystals was different (data not shown), as well as their morphology and composition, showing the differences of formed crystals mainly in accordance with microenvironment composition and isolates.
Interestingly, the nature of the compound obtained was similar in solid and liquid cultures for most isolated marine bacteria. Moreover, the fact that the marine bacterial strains induced CaCO3 precipitation both in presence and absence of urea suggests that several metabolic pathways could be involved in biocalcification, including the urease pathway in presence of urea [12] and anhydrase carbonic pathway in absence of urea [28]. Therefore, we explored whether the bacteria expressed the enzymes involved in these two metabolisms.
 |
Fig. 4 Morphology of crystals observed at the surface of the colonies of marine bacterial strains inducing biomineralization. Marine bacteria grew on solid isolation media for 3 days at 28 °C. The average crystal length depends on the strain. CD7: 30 μm; CD2 and CD8: 50 μm; CD3, SW1 and SW3: 60 μm; CD1: 70 μm; CD4, CD9, CD10, SW2: 90 μm; Md1:100 μm; CD5 and CD6: heterogeneous. Sporosarcina pasteurii DSM 33: 30 μm. |
 |
Fig. 5 Raman spectra of crystals from three bacterial strains: (a) CD9 grown on solid MB medium; (b) Sporosarcina pasteurii DSM33 grown on M220 media; (c) CD8 grown on M1 + CaCl2 + urea. A: aragonite; C: calcite. |
Characterization of the precipitates induced by isolated marine bacteria and Sporosarcina pasteurii DSM 33 as strain model, developed in four solid and liquid culture media, MB + CaCl2, MB + CaCl2 + urea, M1 + CaCl2, M1 + CaCl2 + urea.
3.3 Metabolic pathways involved in biomineralization
Enzymatic colorimetric assay was used to determine presence or absence of urease and carbonic anhydrase activities, enzymatic pathways identified to play a key role in the biomineralization process (Tab. 3).
Using indole urea medium, 6 isolated marine strains were shown to have urease activity: CD6, CD9, CD10, SW1, SW2 and SW3. It is reported that urease pathway in biocalcification is sufficient to induce CaCO3 formation [10]. So, the 6 strains have the enzymatic capacity to use the urea of the culture medium as inducer of the biocalcification which is consistent with the CaCO3 production observed in presence of urea (except for CD6 in solid M1 + CaCl2 + urea, Tab. 2). However, these urease-positive strains and the urease-negative strains produce CaCO3 in absence of urea in the culture medium (Tab. 2) suggesting that another metabolism was involved in biocalcification.
Using p-NPA as a colour indicator during its hydrolysis, we have determined that all isolated marine strains could exhibit carbonic anhydrase activity. This later has been found to have a great potential for production of carbonates [20,21]. Carbonic anhydrase is a major enzyme that plays an important role in carbon concentrating mechanism and sequestration of CO2 into calcium carbonate [29]. It is reported that an extracellular Bacillus carbonic anhydrase produced calcite [28]. This suggests that the carbonic anhydrase pathway alone can induce biocalcification and this may explain the formation of CaCO3 in urease-negative strains and urease-positive strains without urea in the medium. Surprisingly, for DC1 to DC8 strains, the production of CaCO3 only worked in MB + CaCl2 and not in M1 + CaCl2 (in presence or absence of urea). This result could be explained not only by the metabolism of the bacteria but also by the difference of the composition of the culture media. Compound(s) of the M1 medium could interfere with biocalcification.
The presence of carbonic anhydrase activity in the fourteen marine bacteria needs to be confirmed because the carbonic anhydrase assay was conducted without negative control, i.e. without known bacteria issue from the literature that does not exhibit carbonic anhydrase activity. Indeed, carbonic anhydrase enzyme is widespread in prokaryotes [30]. Therefore, it will be necessary in an upcoming study to use another method in order to validate carbonic anhydrase activity of marine bacterial strains.
Both enzymatic pathways, urease and carbonic anhydrase, have often been studied separately as inducing biomineralization [10,15,23,31,32] but it is possible that a coupling of these 2 ways would allow better biocalcification in some bacteria [18,19,33]. The urease pathway would provide an alkalinisation around the cells by hydrolysis of urea and the carbonic anhydrase pathway would provide an additional source of HCO3−.
Based on our results, 6 strains could couple the action of both enzymes: CD6, CD9, CD10, SW1, SW2 and SW3. The synergy of these 2 pathways would allow better efficiency of the precipitation of calcium carbonate.
Urease and carbonic anhydrase assay on isolates marine strains.
3.4 Assessment of biomineralization of Sporosarcina pasteurii DSM 33 by formation of sand-limestone agglomerate in the bio-solid column system
In order to evaluate the ability of the biocalcifying seawater bacteria to agglomerate a sandy matrix with CaCO3, we have developed a bio-solid column system as presented in Figure 2. In order to validate our experimental setup, the urease positive bacterium Sporosarcina pasteurii DSM33 was used as reference strain for biomineralization [23] with the experimental conditions described in Section 2.10. After 3 weeks of treatment in the column system, solid sandy agglomerate was produced (Fig. 6). µ-Raman analyses performed in zones between sand grains revealed the presence of calcite (Fig. 7). This CaCO3, produced by bacteria activity, acted as cement between sand grains. The microorganisms acted as nucleation sites for CaCO3 precipitation and the CaCO3 crystals, as they grew, finally created a bond between the grains of sand. This phenomenon has already been observed in particular when bacteria have previously been brought into contact with sand particles [34].
In this experiment, it was calcite (Fig. 7) that precipitated rather than aragonite, in contrast with what was observed during the biocalcification tests in liquid media M220 + 2% urea by Sporosarcina pasteurii DSM33 (Fig. 5b). Composition of the culture medium is one of the factors that can influence the mineral type produced by biomineralization [27], which could explain why two allotropic forms of CaCO3 could be obtained with the same bacterial strain in two different media. However, several studies demonstrated that Sporosarcina pasteurii produced calcite in similar conditions (sand, cementation medium, mould) with different experimental duration [35–38].
The most compact part of this concretion corresponded to the bottom of the column. Bernardi et al. [35] also reported that more cementation occurred on the inlet side of the cementation medium and gradually decreased throughout the brick length after the biocementation of sand by Sporosarcina pasteurii. The biased cementation at the bottom of the brick was likely due to a higher bacterial concentration at the bottom of the brick since the bacteria were regularly fed with cementation medium from the bottom to the top. We also cannot exclude a problem of sand heterogeneity throughout the column length, which would explain an irregular diffusion of the cementation medium. A piece of 50.2 mm long and 43.2 mm in diameter was then cut by a circular saw and characterized by axial compressive test, accessible water porosity, and thermogravimetric analysis.
The initial intergranular porosity, said to be accessible to water, corresponds to the initial void volume, expressed as a function of the total volume of the sample, which can potentially be filled by CaCO3 during the experiment. This porosity decreases from 40% to 20%, which means that half of the inter-granular voids have been filled by CaCO3 compounds, that corresponds to about 13% of the total mass of the column. The most interesting results was the mechanical compressive strength of this block that reached 4.2 MPa, a resistance similar to that of a hollow concrete block B40 type (compressive strength required of 4 MPa) after only 3 weeks of experiment. These results were slightly better than those obtained by Bernardi et al. [35]. Indeed, they obtained bio-bricks with an optimum compressive strength of 2.2 MPa and a calcite content of 3.4% after 27 days. Other biomineralization studies showed maximum mechanical compressive strengths for bio-bricks of 0.9 MPa [23], 0.9 to 1.1 MPa [37], 2.7 MPa [38] but no values of calcite contents were given by the authors. The variation in compressive strength for the experiments reported here could be due to different liquid flow patterns, surface area of the sand and sand colonization by bacteria [37].
This test of biomineralization with Sporosarcina pasteurii DSM33 by formation of sand-limestone aggregate in the bio-solid column system could be adapted to evaluate the ability of our isolated marine strains to form CaCO3 aggregate.
 |
Fig. 6 Solid sand limestone aggregates obtained after 3 weeks treatment in its entirety (a). The first 5 cm of the column were used for analyses (b). |
 |
Fig. 7 Raman spectrum of the sand-limestone aggregates formed after 3 weeks treatment in the bio-solid column with Sporosarcina pasteurii DSM33. C: calcite. |
4 Conclusion
For the first time, biocalcifying bacterial strains were isolated from calcareous deposits formed on a cathodically protected steel mesh in marine environment. They were shown to have metabolisms responsible for alkalinisation of cell environment. It resulted in CaCO3 precipitation on the cell surface under different conditions. Calcite precipitation was observed in presence and absence of urea, which suggests the involvement of several biocalcification pathways. Among the fourteen isolated strains, six had the enzymatic capacity to use the urea of the culture medium as inducer of the biocalcification whereas all of them would be carbonic anhydrase-positive. The synergy of these 2 metabolic pathways for CD6, CD9, CD10, SW1, SW2 and SW3 strains would improve the precipitation of calcium carbonate.
In parallel, we validated a column biomineralization set-up with a biocalcification model, Sporosarcina pasteurii DSM33. A solid bio-brick with a compressive strength similar to a hollow concrete block was obtained. This experiment can be now adapted to our biocalcifying marine bacteria to investigate their ability to agglomerate a sandy matrix in marine cementation medium.
Conflict of interest
The authors declare that they have no conflicts of interest in relation to this article.
Acknowledgments
Authors want to thank the “Communauté d’agglomération de La Rochelle” (CDA) for the financial support (PhD grant) of Julia Vincent. We also acknowledge the La Rochelle sailing harbour that enabled us to set up an in situ platform for natural seawater experiments.
References
- GéoLittoral, « Indicateur national de l’érosion côtière », 29 janvier 2016, http://www.geolittoral.developpement-durable.gouv.fr/indicateur-national-de-l-erosion-cotiere-r473.html [Google Scholar]
- A. Zanibellato, Synthèse et études physico-chimiques d’un agglomérat calcomagnésien formé sur acier en milieu marin : un éco-matériau pour la protection du littoral, PhD thesis, Université de La Rochelle, 2016 [Google Scholar]
- W.A. Hamilton, Microbially influenced corrosion as a model system for the study of metal microbe interactions: a unifying electron transfer hypothesis, Biofouling 19, 65–76 (2003) [CrossRef] [Google Scholar]
- F. Shiraishi, et al., Cyanobacterial exopolymer properties differentiate microbial carbonate fabrics, Sci. Rep. 7(1), 11805 (2017) [CrossRef] [Google Scholar]
- T. Danjo, S. Kawasaki, Formation mechanisms of beachrocks in Okinawa and Ishikawa, Japan, with a focus on cements, Mater. Trans. 55(3), 493–500 (2014) [CrossRef] [Google Scholar]
- E. Gérard, et al., Key role of Alphaproteobacteria and Cyanobacteria in the formation of stromatolites of Lake Dziani Dzaha (Mayotte, Western Indian Ocean), Front. Microbiol. 9 (2018) [Google Scholar]
- M.J. Castro-Alonso, L.E. Montañez-Hernandez, M.A. Sanchez-Muñoz, M.R. Macias Franco, R. Narayanasamy, N. Balagurusamy, Microbially Induced Calcium Carbonate Precipitation (MICP) and its potential in bioconcrete: microbiological and molecular concepts, Front. Mater. 6, 126 (2019) [CrossRef] [Google Scholar]
- S. Castanier, G. Le Métayer-Levrel, J.-P. Perthuisot, Ca-carbonates precipitation and limestone genesis − the microbiogeologist point of view, Sediment. Geol. 126, 9–23 (1999) [CrossRef] [Google Scholar]
- C. Dupraz, R.P. Reid, O. Braissant, A.W. Decho, R.S. Norman, P.T. Visscher, Processes of carbonate precipitation in modern microbial mats, Earth Sci. Rev. 96(3), 141–162 (2009) [CrossRef] [Google Scholar]
- B. Krajewska, Urease-aided calcium carbonate mineralization for engineering applications: a review, J. Adv. Res. 13, 59–67 (2018) [CrossRef] [Google Scholar]
- Y. Al-Salloum, S. Hadi, H. Abbas, T. Almusallam, M. A. Moslem, Bio-induction and bioremediation of cementitious composites using microbial mineral precipitation − A review, Constr. Build. Mater. 154, 857–876 (2017) [CrossRef] [Google Scholar]
- M. Hosseini, F. Babaha, M.T.Sh. Al-Rubaye, Urease-producing halophilic bacteria isolated from Bahr Al-Milh Salt Lake, Karbala, Iraq, J. Pure Appl. Microbiol. 11(2), 711–716 (2017) [CrossRef] [Google Scholar]
- J. Dick, et al., Bio-deposition of a calcium carbonate layer on degraded limestone by Bacillus species, Biodegradation 17(4), 357–367 (2006) [CrossRef] [PubMed] [Google Scholar]
- N.K. Dhami, Biomineralization of calcium carbonate polymorphs by the bacterial strains isolated from calcareous sites, J. Microbiol. Biotechnol. 23(5), 707–714 (2013) [CrossRef] [Google Scholar]
- X. Zhao, M. Wang, H. Wang, D. Tang, J. Huang, Y. Sun, Study on the remediation of Cd pollution by the biomineralization of urease-producing bacteria, Int. J. Environ. Res. Public. Health 16(2) (2019) [Google Scholar]
- S. Krause, et al., Marine ammonification and carbonic anhydrase activity induce rapid calcium carbonate precipitation, Geochim. Cosmochim. Acta 243, 116–132 (2018) [CrossRef] [Google Scholar]
- S.A. Abo-El-Enein, A.H. Ali, F.N. Talkhan, H.A. Abdel-Gawwad, Utilization of microbial induced calcite precipitation for sand consolidation and mortar crack remediation, HBRC J. 8(3), 185–192 (2012) [CrossRef] [Google Scholar]
- N.K. Dhami, M.S. Reddy, A. Mukherjee, Synergistic role of bacterial urease and carbonic anhydrase in carbonate mineralization, Appl. Biochem. Biotechnol. 172(5), 2552–2561 (2014) [CrossRef] [Google Scholar]
- V. Achal, X. Pan, Characterization of urease and carbonic anhydrase producing bacteria and their role in calcite precipitation, Curr. Microbiol. 62(3), 894–902 (2011) [CrossRef] [Google Scholar]
- B.H. Jo, S.-K. Im, H.J. Cha, Halotolerant carbonic anhydrase with unusual N-terminal extension from marine Hydrogenovibrio marinus as novel biocatalyst for carbon sequestration under high-salt environments, J. CO2 Util. 26, 415–424 (2018) [CrossRef] [Google Scholar]
- A.D. Ghelani, C.B. Bhagat, P.R. Dudhagara, S.V. Gondalia, R.K. Patel, Biomimetic sequestration of CO2 using carbonic anhydrase from calcite encrust forming marine actinomycetes, Sci. Int. 3(2), 48–57 (2015) [CrossRef] [Google Scholar]
- G.A. Silva-Castro, I. Uad, A. Gonzalez-Martinez, A. Rivadeneyra, J. Gonzalez-Lopez, M.A. Rivadeneyra, Bioprecipitation of calcium carbonate crystals by bacteria isolated from saline environments grown in culture media amended with seawater and real brine, BioMed Res. Int. 2015 (2015) [Google Scholar]
- J. Henze, D.G. Randall, Microbial induced calcium carbonate precipitation at elevated pH values (>11) using Sporosarcina pasteurii, J. Environ. Chem. Eng. 6(4), 5008–5013 (2018) [CrossRef] [Google Scholar]
- H. Agogué, D. Lamy, P.R. Neal, M.L. Sogin, G.J. Herndl, Water mass-specificity of bacterial communities in the North Atlantic revealed by massively parallel sequencing, Mol. Ecol. 20(2), 258–274 (2011) [CrossRef] [Google Scholar]
- L. Chen, et al., Variation in microbial community structure in surface seawater from Pearl River Delta: discerning the influencing factors, Sci. Total Environ. 660, 136–144 (2019) [CrossRef] [Google Scholar]
- C. Jimenez-Lopez, C. Rodriguez-Navarro, G. Piñar, F.J. Carrillo-Rosúa, M. Rodriguez-Gallego, M.T. Gonzalez-Muñoz, Consolidation of degraded ornamental porous limestone stone by calcium carbonate precipitation induced by the microbiota inhabiting the stone, Chemosphere 68(10), 1929–1936 (2007) [CrossRef] [Google Scholar]
- A. López-Moreno, J.D. Sepúlveda-Sánchez, E.M. Mercedes Alonso Guzmán, S. Le Borgne, Calcium carbonate precipitation by heterotrophic bacteria isolated from biofilms formed on deteriorated ignimbrite stones: influence of calcium on EPS production and biofilm formation by these isolates, Biofouling 30(5), 547–560 (2014) [CrossRef] [Google Scholar]
- S. Sundaram, I.S. Thakur, Induction of calcite precipitation through heightened production of extracellular carbonic anhydrase by CO2 sequestering bacteria, Bioresour. Technol. 253, 368–371 (2018) [CrossRef] [Google Scholar]
- A. Kaplan, L. Reinhold, CO2 concentrating mechanisms in photosynthetic microorganisms, Annu. Rev. Plant Physiol. Plant Mol. Biol. 50(1), 539–570 (1999) [CrossRef] [Google Scholar]
- K.S. Smith, J.G. Ferry, Prokaryotic carbonic anhydrases, FEMS Microbiol. Rev. 24(4), 335–366 (2000) [CrossRef] [Google Scholar]
- G.M. Bond, J. Stringer, D.K. Brandvold, F.A. Simsek, M.-G. Medina, G. Egeland, Development of integrated system for biomimetic CO2 sequestration using the enzyme carbonic anhydrase, Energy Fuels 15(2), 309–316 (2001) [CrossRef] [Google Scholar]
- N. Favre, M.L. Christ, A.C. Pierre, Biocatalytic capture of CO2 with carbonic anhydrase and its transformation to solid carbonate, J. Mol. Catal. B Enzym. 60(3–4), 163–170 (2009) [CrossRef] [Google Scholar]
- J.N. Priya, M.K. Nan, Effect of carbonic anhydrase and urease on bacterial Calcium carbonate precipitation, Int. J. Pharma Bio Sci. 8(3) (2017) [CrossRef] [Google Scholar]
- F. Hammes, W. Verstraete, Key roles of pH and calcium metabolism in microbial carbonate precipitation, Rev. Environ. Sci. Biotechnol. 1(1), 3–7 (2002) [CrossRef] [Google Scholar]
- D. Bernardi, J.T. DeJong, B.M. Montoya, B.C. Martinez, Bio-bricks: biologically cemented sandstone bricks, Constr. Build. Mater. 55, 462–469 (2014) [CrossRef] [Google Scholar]
- H.-J. Chen, Y.-H. Huang, C.-C. Chen, J. P. Maity, C.-Y. Chen, Microbial Induced Calcium Carbonate Precipitation (MICP) using pig urine as an alternative to industrial urea, Waste Biomass Valoriz. 10(10), 2887–2895 (2019) [CrossRef] [Google Scholar]
- S.G. Choi, J. Chu, R.C. Brown, K. Wang, Z. Wen, Sustainable biocement production via microbially induced calcium carbonate precipitation: use of limestone and acetic acid derived from pyrolysis of lignocellulosic biomass, ACS Sustain. Chem. Eng. 5(6), 5183–5190 (2017) [CrossRef] [Google Scholar]
- S.E. Lambert, D.G. Randall, Manufacturing bio-bricks using microbial induced calcium carbonate precipitation and human urine, Water Res. 160, 158–166 (2019) [CrossRef] [Google Scholar]
Cite this article as: Julia Vincent, René Sabot, Isabelle Lanneluc, Philippe Refait, Philippe Turcry, Pierre-Yves Mahieux, Marc Jeannin, Sophie Sablé, Biomineralization of calcium carbonate by marine bacterial strains isolated from calcareous deposits, Matériaux & Techniques 108, 302 (2020)
All Tables
Characterization of isolated bacterial strains inducing mineral agglomerates on the surface of the colonies.
Characterization of the precipitates induced by isolated marine bacteria and Sporosarcina pasteurii DSM 33 as strain model, developed in four solid and liquid culture media, MB + CaCl2, MB + CaCl2 + urea, M1 + CaCl2, M1 + CaCl2 + urea.
All Figures
 |
Fig. 1 Experimental procedure for obtaining bacteria able to carry out biomineralization in the marine environment (A) and setting up a reactor for manufacturing bio-bricks (B). X: no colonies with mineral precipitation on their surface. |
| In the text | |
 |
Fig. 2 Set-up of the column reactor adapted from Henze and Randall [23]. |
| In the text | |
 |
Fig. 3 Colonies of marine bacterial strains with mineral crust on the surface of the colonies. CD1 to CD8 were isolated on solid MB + CaCl2, other bacteria were isolated on solid M1 + CaCl2 for 3 days at 25 °C. Arrows indicate mineral agglomerate induced by bacteria. |
| In the text | |
 |
Fig. 4 Morphology of crystals observed at the surface of the colonies of marine bacterial strains inducing biomineralization. Marine bacteria grew on solid isolation media for 3 days at 28 °C. The average crystal length depends on the strain. CD7: 30 μm; CD2 and CD8: 50 μm; CD3, SW1 and SW3: 60 μm; CD1: 70 μm; CD4, CD9, CD10, SW2: 90 μm; Md1:100 μm; CD5 and CD6: heterogeneous. Sporosarcina pasteurii DSM 33: 30 μm. |
| In the text | |
 |
Fig. 5 Raman spectra of crystals from three bacterial strains: (a) CD9 grown on solid MB medium; (b) Sporosarcina pasteurii DSM33 grown on M220 media; (c) CD8 grown on M1 + CaCl2 + urea. A: aragonite; C: calcite. |
| In the text | |
 |
Fig. 6 Solid sand limestone aggregates obtained after 3 weeks treatment in its entirety (a). The first 5 cm of the column were used for analyses (b). |
| In the text | |
 |
Fig. 7 Raman spectrum of the sand-limestone aggregates formed after 3 weeks treatment in the bio-solid column with Sporosarcina pasteurii DSM33. C: calcite. |
| In the text | |
Les statistiques affichées correspondent au cumul d'une part des vues des résumés de l'article et d'autre part des vues et téléchargements de l'article plein-texte (PDF, Full-HTML, ePub... selon les formats disponibles) sur la platefome Vision4Press.
Les statistiques sont disponibles avec un délai de 48 à 96 heures et sont mises à jour quotidiennement en semaine.
Le chargement des statistiques peut être long.